37275
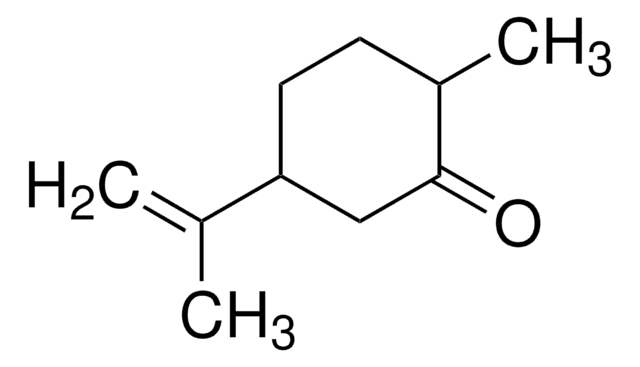
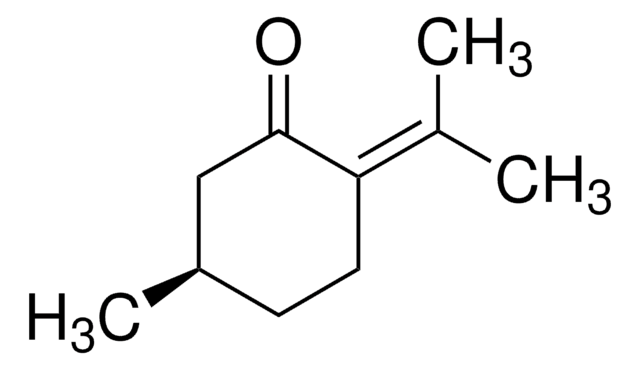
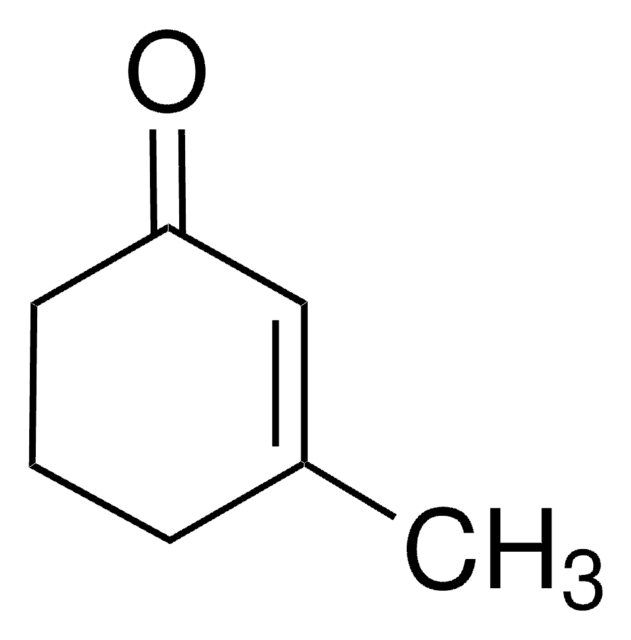
(+)-Dihydrocarvone
mixture of isomers
Sinónimos:
(2R,5R)-5-Isopropenyl-2-methylcyclohexanone
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C10H16O
Número de CAS:
Peso molecular:
152.23
Beilstein/REAXYS Number:
2044615
EC Number:
MDL number:
UNSPSC Code:
12352115
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
form
liquid
optical activity
[α]20/D +20±2°, neat
composition
n-(+)-dihydrocarvone, ~77%
iso-(+)-dihydrocarvone, ~20%
refractive index
n20/D 1.471
density
0.928 g/mL at 20 °C (lit.)
SMILES string
CC1CCC(CC1=O)C(C)=C
InChI
1S/C10H16O/c1-7(2)9-5-4-8(3)10(11)6-9/h8-9H,1,4-6H2,2-3H3
InChI key
AZOCECCLWFDTAP-UHFFFAOYSA-N
General description
(+)-Dihydrocarvone, a monoterpenoid compound found in caraway oil, is a key building block to synthesize sesquiterpenes. It is generally produced either by the hydrogenation of carvone or oxidation of limonene.
Application
(+)-Dihydrocarvone may be used in the following processes:
- Synthesis of dispiro 1,2,4,5-tetraoxanes, which show potent anti-malarial activity.
- Synthesis of an epoxylactone by oxidation, which can undergo copolymerization with ε-caprolactone to form cross-linked copolymers with shape memory properties.
- Synthesis of α-Cyperone, a eudesmane type sesquiterpenoid compound with potent insecticidal activity.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
215.6 °F - closed cup
flash_point_c
102 °C - closed cup
ppe
Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Insecticidal activity of sesquiterpenes skeleton synthesized by the conventional Robinson annulations reaction on Drosophila melanogaster.
Alarcon J, et al.
Industrial Crops and Products, 42, 268-272 (2013)
Oxidized dihydrocarvone as a renewable multifunctional monomer for the synthesis of shape memory polyesters.
Lowe JR, et al.
Biomacromolecules, 10(7), 2003-2008 (2009)
The structure and antimalarial activity of dispiro-1, 2, 4, 5-tetraoxanes derived from (+)-dihydrocarvone.
Dong Y, et al.
Bioorganic & Medicinal Chemistry Letters, 20(22), 6359-6361 (2010)
N-functionalization of dihydrocarvone: Obtaining aminocyclohexane derivatives and their spectrometric study.
Kouznetsov VV and Stashenko EE.
Journal of the Chilean Chemical Society, 50(3), 559-563 (2005)
A Convenient Procedure for the Preparation of Dihydrocarvone.
Raucher S and Hwang K-J.
Synthetic Communications, 10(2), 133-137 (1980)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico