273937
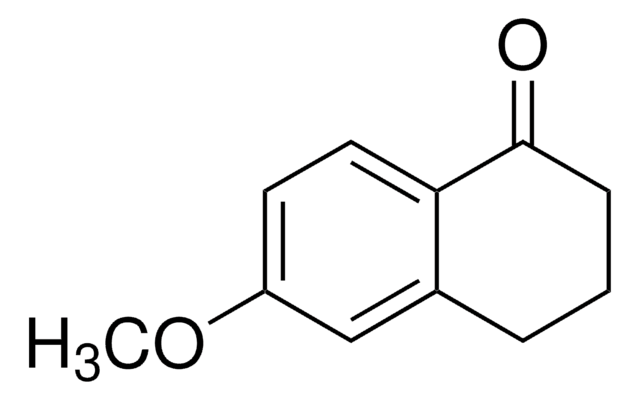
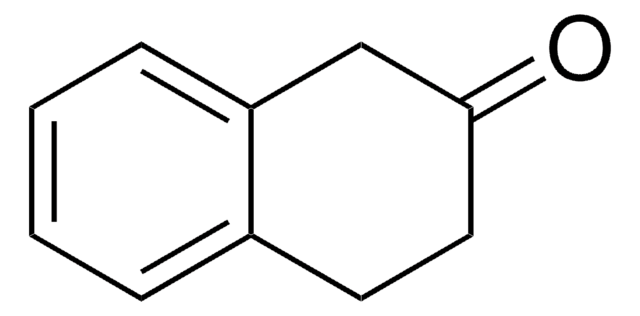
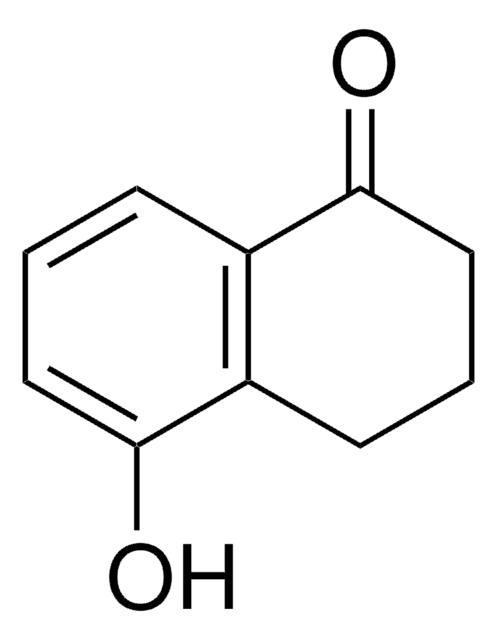
6,7-Dimethoxy-1-tetralone
97%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C12H14O3
Número de CAS:
Peso molecular:
206.24
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
97%
form
powder
mp
98-100 °C (lit.)
functional group
ketone
SMILES string
COc1cc2CCCC(=O)c2cc1OC
InChI
1S/C12H14O3/c1-14-11-6-8-4-3-5-10(13)9(8)7-12(11)15-2/h6-7H,3-5H2,1-2H3
InChI key
YNNJHKOXXBIJKK-UHFFFAOYSA-N
General description
6,7-Dimethoxy-1-tetralone reacts with 2-amino-4,5-dimethoxyacetophenone to form 5,6-dihydro-2,3,9,10-tetramethoxybenz[c]acridine.
Application
6,7-Dimethoxy-1-tetralone was used in the synthesis of 2-bromotetralones by undergoing bromination. It was also used as a precursor to quinolines with dopaminergic activity, naphthols with anti-inflammatory activity and benzophenanthridine alkaloids with antitumor activity.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Colin J Dunsmore et al.
Bioorganic & medicinal chemistry letters, 18(5), 1730-1734 (2008-02-12)
Several 2-aminotetralones were identified as novel inhibitors of the bacterial enzymes MurA and MurZ. A number of these inhibitors demonstrated antibacterial activity against Staphylococcus aureus and Escherichia coli with MICs in the range 8-128 microg/ml. Based on structure-activity relationships we
D G Batt et al.
Journal of medicinal chemistry, 33(1), 360-370 (1990-01-01)
The synthesis, biological evaluation, and structure-activity relationships of a series of 1-naphthols bearing carbon substituents at the 2-position are described. These compounds are potent inhibitors of the 5-lipoxygenase from RBL-1 cells and also inhibit bovine seminal vesicle cyclooxygenase. Structure-activity relationships
The Journal of Organic Chemistry, 57, 5907-5907 (1992)
J C Craig et al.
Journal of medicinal chemistry, 32(5), 961-968 (1989-05-01)
A series of 2-substituted octahydrobenzo[f]quinolines has been synthesized and assayed for dopamine agonist activity. Only the compounds corresponding to the beta-rotameric conformation of dopamine showed biphasic activity in competition binding studies with the radioligand [3H]spiroperidol. These findings suggest that the
D Makhey et al.
Bioorganic & medicinal chemistry, 8(5), 1171-1182 (2000-07-06)
Coralyne and several other synthetic benzo[a,g]quinolizium derivatives related to protoberberine alkaloids have exhibited activity as topoisomerase poisons. These compounds are characterized by the presence of a positively charged iminium group, which has been postulated to be associated with their pharmacological
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico