244988
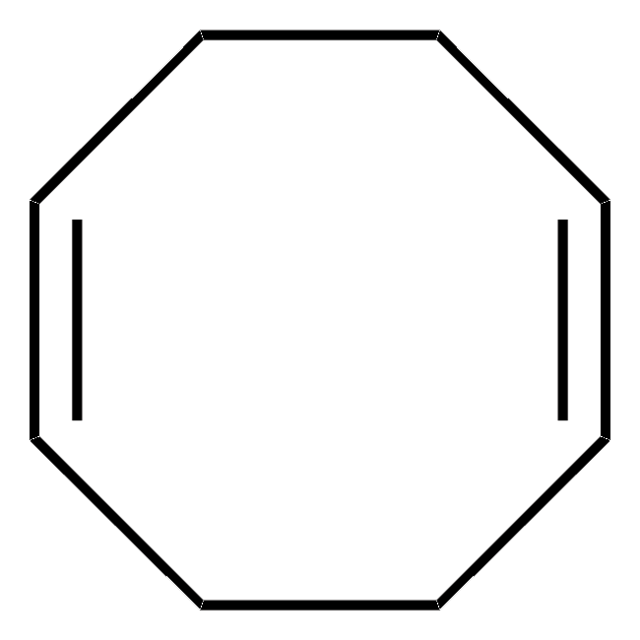
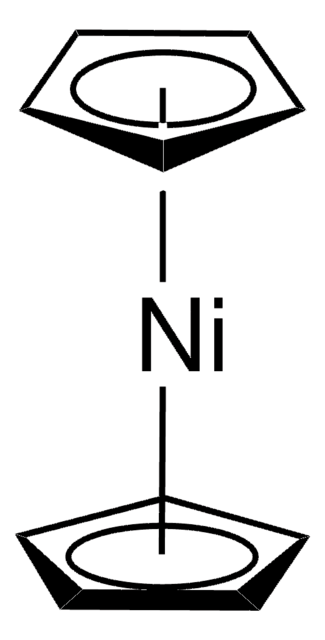
Bis(1,5-cyclooctadiene)nickel(0)
Sinónimos:
Bis(cyclooctadiene)nickel, Ni(COD)2
About This Item
Productos recomendados
Quality Level
reaction suitability
core: nickel
reaction type: Cross Couplings
reagent type: catalyst
parameter
temperature sensitive
mp
60 °C (dec.) (lit.)
storage temp.
−20°C
SMILES string
[Ni].C1CC=CCCC=C1.C2CC=CCCC=C2
InChI
1S/2C8H12.Ni/c2*1-2-4-6-8-7-5-3-1;/h2*1-2,7-8H,3-6H2;/b2*2-1-,8-7-;
InChI key
JRTIUDXYIUKIIE-KZUMESAESA-N
Categorías relacionadas
Application
- Oxidative addition reactions
Catalyst for:
- Asymmetric α-arylation and heteroarylation of ketones with chloroarenes
- Cross-coupling reactions
- Regioselective and stereoselective carboxylation/cyclization of allenyl aldehydes under a carbon dioxide atmosphere
- Methyl carboxylation of homopropargylic alcohols
- Stereoselective borylative ketone-diene coupling
- Cycloaddition of benzamides with internal alkynes
Related product
signalword
Danger
hcodes
Hazard Classifications
Carc. 2 - Flam. Sol. 1 - Skin Sens. 1 - STOT RE 1
target_organs
Lungs
Storage Class
4.1B - Flammable solid hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Csp2- and Csp-hybridized coupling reactions are established catalytic approaches. However, multi-step Csp3- and Csp2-coupling reactions of boronic acids and related derivatives are still limited by ineffective two-electron transmetalation reactions.
Nickel transition metal and its complexes can be used as a catalyst in many synthetic transformations, like oxidative addition, C-H activation, reductive elimination, oxidative cyclization, oligomerization, and in cross-coupling reactions.
Global Trade Item Number
| Número de referencia del producto (SKU) | GTIN |
|---|---|
| 244988-2G | 4061825772400 |
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico