223220
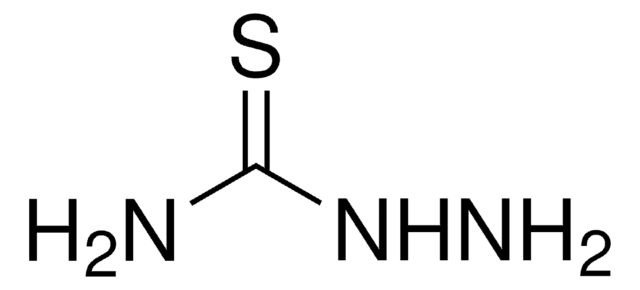
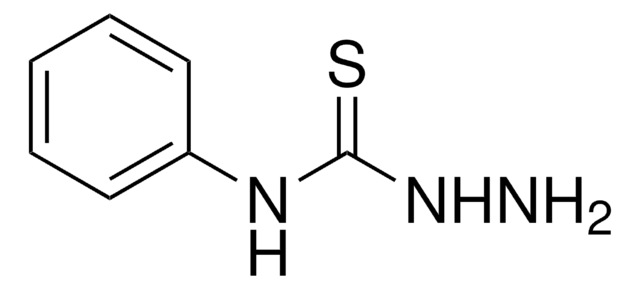
Thiocarbohydrazide
98%
Sinónimos:
Thiocarbonyldihydrazide
About This Item
Productos recomendados
Quality Level
assay
98%
form
solid
mp
171-174 °C (dec.) (lit.)
functional group
amine
hydrazine
thiourea
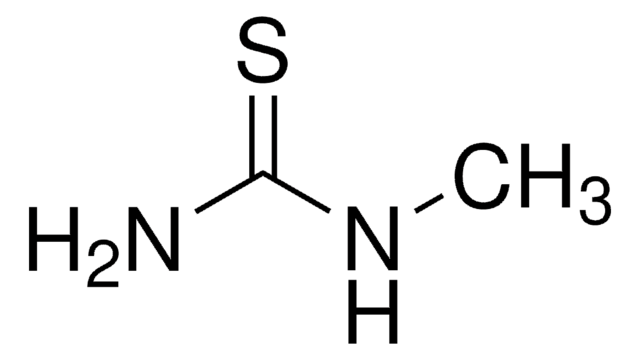
SMILES string
NNC(=S)NN
InChI
1S/CH6N4S/c2-4-1(6)5-3/h2-3H2,(H2,4,5,6)
InChI key
LJTFFORYSFGNCT-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- Review of transition metal complexes with thiocarbohydrazides: This comprehensive review discusses the coordination chemistry of thiocarbohydrazides with various metals, highlighting their relevance in the synthesis of complex metal compounds used in catalysis and pharmaceutical research (Aly et al., 2023).
- Pd-doped nanocomposites for organometallic catalysis: Thiocarbohydrazide was employed in the fabrication of Pd-doped SBA-15 nanocomposites, applied as catalysts in the synthesis of organometallic compounds, showcasing its utility as a catalyst support material (Kalhor and Dadras, 2021).
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 2 Oral
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico