201014
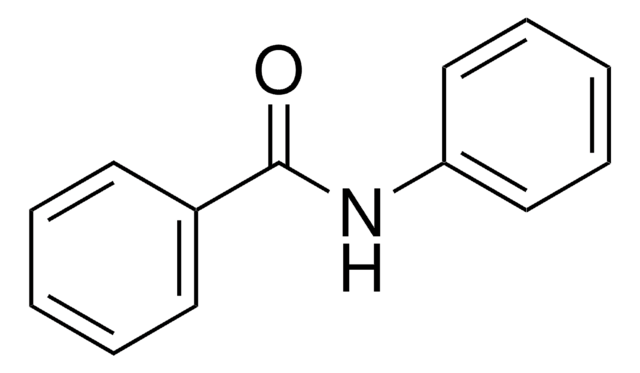
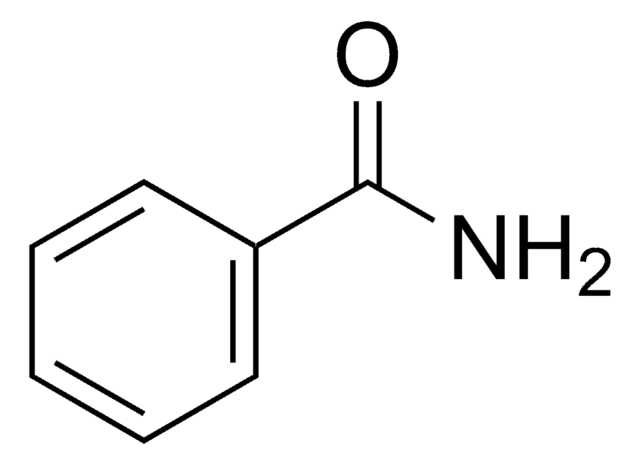
N-Benzylbenzamide
≥98%
Sinónimos:
Benzoic acid benzylamide, N-(Phenylmethyl)benzamide, N-Benzoylbenzylamine
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
C6H5CONHCH2C6H5
Número de CAS:
Peso molecular:
211.26
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
≥98%
mp
104-106 °C (lit.)
solubility
acetone: 25 mg/mL, clear, colorless
functional group
amide
phenyl
SMILES string
O=C(NCc1ccccc1)c2ccccc2
InChI
1S/C14H13NO/c16-14(13-9-5-2-6-10-13)15-11-12-7-3-1-4-8-12/h1-10H,11H2,(H,15,16)
InChI key
LKQUCICFTHBFAL-UHFFFAOYSA-N
General description
N-Benzylbenzamide inhibits the activity of tyrosinase.
Application
A convenient precursor to α-substituted benzylamines and an indicator for the titration of butyllithium and other lithium bases. For references see Aldrichimica Acta .
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Aldrichimica Acta, 11, 20-20 (1978)
Yae Eun Chong et al.
Biomedical chromatography : BMC, 33(11), e4653-e4653 (2019-07-20)
Ondansetron, a widely used antiemetic agent, is a P-glycoprotein (P-gp) substrate and therefore expression of P-gp at the blood-brain barrier limits its distribution to the central nervous system (CNS), which was observed to be reversed by coadministration with P-gp inhibitors.
Zsanett Dorkó et al.
Talanta, 162, 167-173 (2016-11-14)
A simple and efficient method is presented for assessing molecularly imprinted polymers (MIP) and other sorbents from the point of view of practical applications. The adsorption isotherms of the compounds, which need to be separated or detected in an application
Sung Jin Cho et al.
Bioorganic & medicinal chemistry letters, 16(10), 2682-2684 (2006-03-04)
A series of potent inhibitors of tyrosinase and their structure-activity relationships are described. N-Benzylbenzamide derivatives (1-21) with hydroxyl(s) were synthesized and tested for their tyrosinase inhibitory activity. With this series, compound 15 provided a potent tyrosinase inhibition: it effectively inhibited
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico