187062
3-Bromobenzyl bromide
99%
Sinónimos:
α,3-Dibromotoluene
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
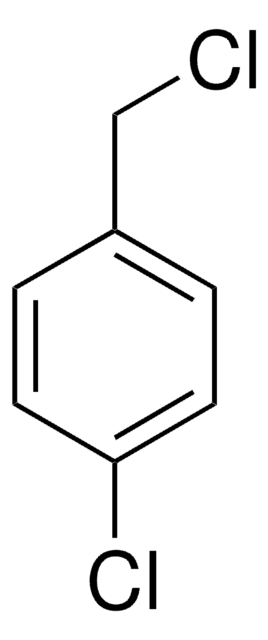
Fórmula lineal:
BrC6H4CH2Br
Número de CAS:
Peso molecular:
249.93
Beilstein/REAXYS Number:
2078683
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
99%
form
solid
mp
39-41 °C (lit.)
SMILES string
BrCc1cccc(Br)c1
InChI
1S/C7H6Br2/c8-5-6-2-1-3-7(9)4-6/h1-4H,5H2
InChI key
ZPCJPJQUVRIILS-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
3-Bromobenzyl bromide undergoes reduction with diethylzinc in the presence of Pd(PPh3)4 to yield corresponding hydrocarbon.
Application
3-Bromobenzyl bromide was used in the synthesis of:
- 1,7-di(3-bromobenzyl)cyclen
- substituted 8-arylquinoline, phosphodiesterase 4 (PDE4) inhibitors
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Synthesis of a new family of bi-and polycyclic compounds via Pd-catalyzed amination of 1, 7-di (3-bromobenzyl) cyclen.
Averin AD, et al.
Tetrahedron Letters, 49(24), 3950-3954 (2008)
Reduction of benzylic halides with diethylzinc using tetrakis (triphenylphosphine) palladium as catalyst.
Agrios KA and Srebnik M.
The Journal of Organic Chemistry, 58(24), 6908-6910 (1993)
Dwight Macdonald et al.
Bioorganic & medicinal chemistry letters, 15(23), 5241-5246 (2005-09-20)
The discovery and SAR of a new series of substituted 8-arylquinoline PDE4 inhibitors are herein described. This work has led to the identification of several compounds with excellent in vitro and in vivo profiles, including a good therapeutic window of
William L Scott et al.
Journal of combinatorial chemistry, 11(1), 14-33 (2008-12-25)
Distributed Drug Discovery (D(3)) proposes solving large drug discovery problems by breaking them into smaller units for processing at multiple sites. A key component of the synthetic and computational stages of D(3) is the global rehearsal of prospective reagents and
William L Scott et al.
Journal of combinatorial chemistry, 11(1), 34-43 (2008-12-25)
For the successful implementation of Distributed Drug Discovery (D(3)) (outlined in the accompanying Perspective), students, in the course of their educational laboratories, must be able to reproducibly make new, high quality, molecules with potential for biological activity. This article reports
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico