16490
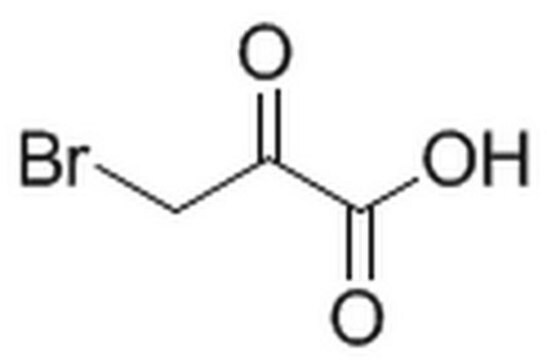
Bromopyruvic acid
≥98.0%
Sinónimos:
3-Bromo-2-oxopropionic acid
About This Item
Productos recomendados
Quality Level
assay
≥98.0%
form
(Powder or Crystals or Flakes)
mp
77-82 °C
solubility
water: soluble 1 g/10 mL, clear to very slightly hazy, colorless
functional group
bromo
carboxylic acid
ketone
storage temp.
2-8°C
SMILES string
OC(=O)C(=O)CBr
InChI
1S/C3H3BrO3/c4-1-2(5)3(6)7/h1H2,(H,6,7)
InChI key
PRRZDZJYSJLDBS-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
We presents an article about the Warburg effect, and how it is the enhanced conversion of glucose to lactate observed in tumor cells, even in the presence of normal levels of oxygen. Otto Heinrich Warburg demonstrated in 1924 that cancer cells show an increased dependence on glycolysis to meet their energy needs, regardless of whether they were well-oxygenated or not.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico