158461
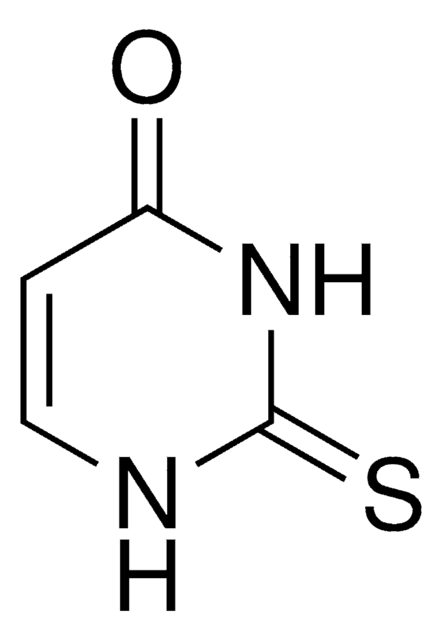
Dithiouracil
98%
Sinónimos:
2,4(1H,3H)-Pyrimidinedithione, 2,4-Dimercaptopyrimidine
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C4H4N2S2
Número de CAS:
Peso molecular:
144.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Productos recomendados
Quality Level
assay
98%
mp
279-281 °C (dec.) (lit.)
SMILES string
S=C1NC=CC(=S)N1
InChI
1S/C4H4N2S2/c7-3-1-2-5-4(8)6-3/h1-2H,(H2,5,6,7,8)
InChI key
ZEQIWKHCJWRNTH-UHFFFAOYSA-N
General description
Dithiouracil is a potential anticancer drug and its redox mechanism and electronic absorption behavior has been investigated in a wide pH range by UV-Vis spectroscopy, cyclic voltammetry and differential pulse voltammetry.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Application of 2-thiouracil and 2,4-dithiouracil for the determination of metal ions. Part I. Spectrophotometric determination of copper and silver.
G Misztal et al.
Annales Universitatis Mariae Curie-Sklodowska. Sectio D: Medicina, 38, 135-141 (1983-01-01)
Afzal Shah et al.
Journal of photochemistry and photobiology. B, Biology, 117, 269-277 (2012-11-06)
The redox mechanism and electronic absorption behavior of a commercial anticancer drug, 5-fluorouracil (5-FU) and two potential anticancer drugs, 2-thiouracil (2-TU) and dithiouracil (DTU) have been investigated in a wide pH range by UV-Vis spectroscopy, cyclic voltammetry and differential pulse
Marvin Pollum et al.
ChemMedChem, 13(10), 1044-1050 (2018-03-14)
Sulfur-substituted nucleobases (i.e., thiobases) are a prospective class of compounds for clinical and cosmetic topical phototherapies. Recent investigations of several thiobases have revealed the ultrafast and efficient population of reactive triplet states upon ultraviolet-A (UVA) irradiation and the subsequent generation
Wen-Jwu Wang et al.
Organic & biomolecular chemistry, 3(16), 3054-3058 (2005-09-28)
2,4-dithiouracil (DTU) forms in the crystals the H-bonded monohydrates of a 1:1:1 ratio with 18-crown-6 (18C6) 1, cis,syn,cis-isomer of dicyclohexano-18-crown-6 (DCH6A) 2, and benzo-18-crown-6 (B18C6) 3, while the anhydrous adduct with cis,anti,cis-isomer of dicyclohexano-18-crown-6 (DCH6B) 4 is of a 2:1
L Lapinski et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 54A(5), 685-693 (1998-07-29)
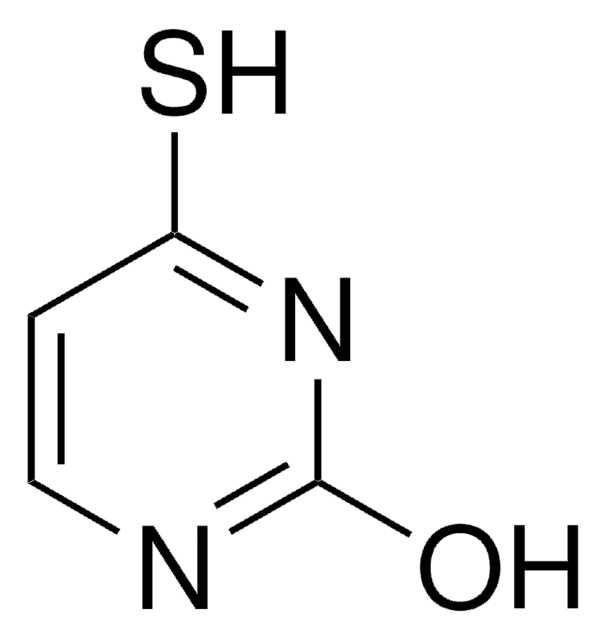
2,4-Pyrimidinedithiol (the dithiol form of 2,4-dithiouracil) was generated by UV (lambda > 335 nm) irradiation of the dithione form of 2,4-dithiouracil isolated in low-temperature argon or nitrogen matrices. The IR and UV spectra of the photoproduct are reported. The dithiol
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico