111279
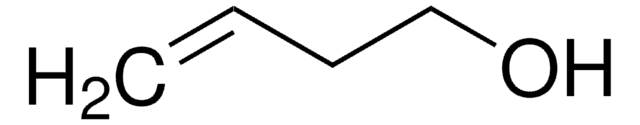
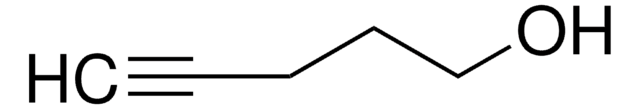
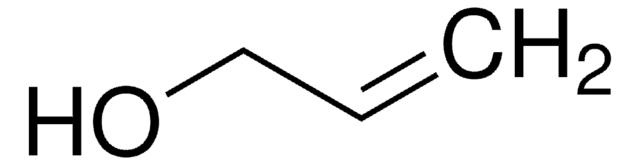
4-Penten-1-ol
99%
Sinónimos:
2-Allylethyl alcohol
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CH2=CH(CH2)3OH
Número de CAS:
Peso molecular:
86.13
Beilstein/REAXYS Number:
1560163
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39020310
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
99%
refractive index
n20/D 1.429 (lit.)
bp
134-137 °C (lit.)
density
0.834 g/mL at 25 °C (lit.)
SMILES string
OCCCC=C
InChI
1S/C5H10O/c1-2-3-4-5-6/h2,6H,1,3-5H2
InChI key
LQAVWYMTUMSFBE-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
4-penten-1-ol forms ester bond at the C terminus of the linear peptide in solution with HATU as coupling agent.
Application
4-Penten-1-ol can be used as a reactant to prepare sulfamate ester by reacting with chlorosulfonyl isocycanate (142662).The derived ester undergoes an enantioselective intramolecular azridination reaction in the presence of Cu catalyst. 4-Penten-1-ol can also be used to study the epoxidation of olefins with oxo-diperoxo tungstate(VI) complex as catalyst and bicarbonate as co-catalyst.
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Enantioselective Intramolecular Copper-Catalyzed Aziridination of Sulfamates
Audrey Esteoule.et al.
Synthesis, 1251-1251 (2007)
Highly efficient epoxidation method of olefins with hydrogen peroxide as terminal oxidant, bicarbonate as a co-catalyst and oxodiperoxo molybdenum(VI) complex as catalyst.
Maiti SK, et al.
New. J. Chem., 30(3), 479-489 (2006)
Stefania Terracciano et al.
Bioorganic & medicinal chemistry, 16(13), 6580-6588 (2008-05-30)
In the recent years, we focused our attention on the cyclodepsipeptide Jaspamide 1, an interesting marine metabolite, possessing a potent inhibitory activity against breast and prostate cancer, as a consequence of its ability to disrupt actin cytoskeleton dynamics. Although its
Marina D Rvovic et al.
Journal of molecular modeling, 17(6), 1251-1257 (2010-08-17)
The mechanism of phenylselenoetherification of pent-4-en-1-ol using some bases (pyridine, triethylamine, quinoline, 2,2'-bipyridine) as catalyst was examined through studies of kinetics of the cyclization, by UV-VIS spectrophotometry. It was demonstrated that the intramolecular cyclization is facilitated in the presence of
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico