推薦產品
等級
pharmaceutical primary standard
API 家族
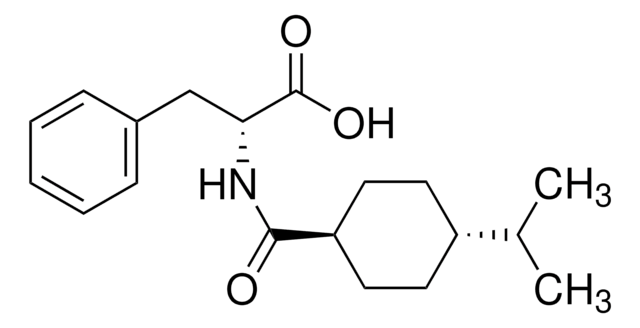
nateglinide
製造商/商標名
USP
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
CC(C)[C@@H]1CC[C@H](CC1)C(=O)N[C@H](Cc2ccccc2)C(O)=O
InChI
1S/C19H27NO3/c1-13(2)15-8-10-16(11-9-15)18(21)20-17(19(22)23)12-14-6-4-3-5-7-14/h3-7,13,15-17H,8-12H2,1-2H3,(H,20,21)(H,22,23)/t15-,16-,17-/m1/s1
InChI 密鑰
OELFLUMRDSZNSF-BRWVUGGUSA-N
基因資訊
human ... ABCC8(6833) , KCNJ11(3767)
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
生化/生理作用
Nateglinide is a Kir6.2/SUR1 channel inhibitor and antidiabetic.
Nateglinide is a Kir6.2/SUR1 channel inhibitor and antidiabetic. It is selective for the SUR1 subtype, which is found on pancreatic islet cells. Nateglinide evokes KATP channel-dependent insulin secretion (50-200 μM) in the absence and presence of insulin.
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
[Effects of nateglinide in impaired glucose tolerance subjects].
Takahisa Hirose
Nihon rinsho. Japanese journal of clinical medicine, 63 Suppl 2, 438-443 (2005-03-23)
I W Campbell
International journal of clinical practice, 59(10), 1218-1228 (2005-09-24)
Therapy for type 2 diabetes mellitus should aim to control not only fasting, but also postprandial glucose levels. Nateglinide, a d-phenylalanine derivative, restores postprandial early phase insulin secretion in a transient and glucose-sensitive manner without affecting basal insulin levels. As
Marc K Israel et al.
Vascular health and risk management, 4(6), 1167-1178 (2008-01-01)
The increasing prevalence of type 2 diabetes provides impetus for both development of new drugs to improve glycemic control and for reconsideration of treatment strategies with existing agents. Combination therapy with complementary drug classes that act on different aspects of
T Ikenoue et al.
Nihon yakurigaku zasshi. Folia pharmacologica Japonica, 116(3), 171-180 (2000-10-14)
An early defect in Type 2 diabetes is the loss of acute insulin release after food intake, which causes prolonged elevation of postprandial glucose levels. Suppressing postprandial hyperglycemia is considered to be very important for preventing diabetic complications. Sulfonylureas are
L S Phillips et al.
International journal of clinical practice, 57(6), 535-541 (2003-08-16)
Nateglinide is a new oral antidiabetic agent that stimulates insulin release promptly after its pre-meal administration in a strongly glucose-dependent fashion. Because its insulinotropic effects are short in duration, nateglinide specifically targets postprandial hyperglycaemia with a low potential to elicit
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務