推薦產品
等級
pharmaceutical primary standard
API 家族
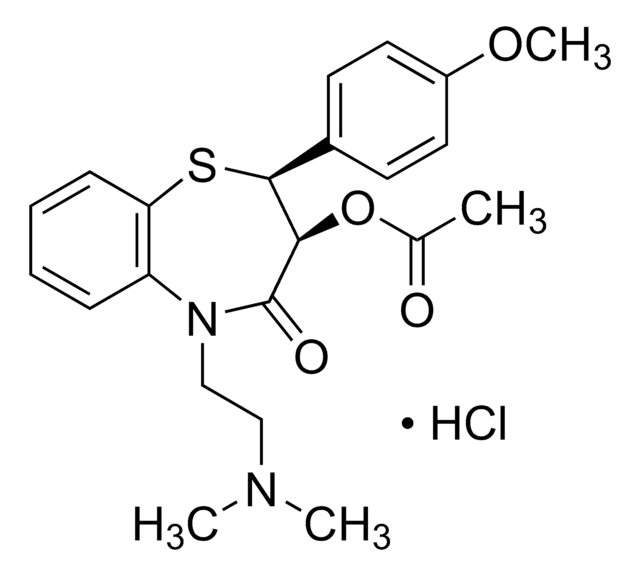
diltiazem
製造商/商標名
USP
應用
pharmaceutical (small molecule)
格式
neat
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Desacetyl diltiazem hydrochloride USP reference standard suitable for use in specified USP compendial tests and assays.
Also used to prepare standard, and system suitability solutions for assay, and impurity analysis according to the given below monographs of United States Pharmacopeia (USP):
Also used to prepare standard, and system suitability solutions for assay, and impurity analysis according to the given below monographs of United States Pharmacopeia (USP):
- Diltiazem Hydrochloride
- Diltiazem Hydrochloride Tablets
- Fluconazole
- Diltiazem Hydrochloride Extended-Release Capsules
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務