1224981
USP
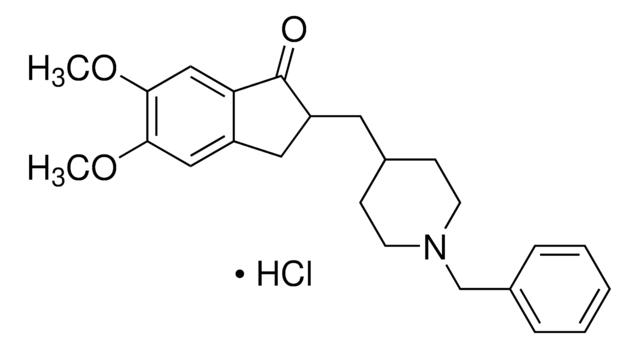
Donepezil hydrochloride
United States Pharmacopeia (USP) Reference Standard
同義詞:
(±)-2-[(1-Benzyl-4-piperidyl)methyl]-5,6-dimethoxy-1-indanone hydrochloride, 2,3-Dihydro-5,6-dimethoxy-2-{[1-(phenylmethyl)-4-piperidinyl]methyl}-1H-inden-1-one
登入查看組織和合約定價
全部照片(1)
About This Item
經驗公式(希爾表示法):
C24H29NO3 · HCl
CAS號碼:
分子量::
415.95
MDL號碼:
分類程式碼代碼:
41116107
NACRES:
NA.24
推薦產品
等級
pharmaceutical primary standard
API 家族
donepezil
製造商/商標名
USP
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
Cl.N2(CCC(CC2)CC3Cc4c(cc(c(c4)OC)OC)C3=O)Cc1ccccc1
InChI
1S/C24H29NO3.ClH/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18;/h3-7,14-15,17,20H,8-13,16H2,1-2H3;1H
InChI 密鑰
XWAIAVWHZJNZQQ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 2 Oral - Eye Irrit. 2
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
F M Garlich et al.
Clinical toxicology (Philadelphia, Pa.), 52(4), 291-294 (2014-04-17)
Donepezil is a centrally-acting, reversible acetylcholinesterase inhibitor that is used in the treatment of Alzheimer disease. Altered mental status, nausea, vomiting, and bradycardia have been reported in therapeutic and supratherapeutic ingestions of donepezil, though pediatric exposures have not been well-described.
Kenneth Rockwood et al.
The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques, 40(4), 564-571 (2013-06-22)
vascular dementia (VaD) and mixed Alzheimer's disease (AD/VaD) are common. How best to monitor treatment is not clear. Our objective was to compare responsiveness and construct validity of change scores, following donepezil treatment, of the standardized Mini-Mental State Examination (sMMSE)
Koteswara Rao Valasani et al.
Journal of chemical information and modeling, 53(8), 2033-2046 (2013-06-20)
Acetylcholinesterase (AChE) is a main drug target, and its inhibitors have demonstrated functionality in the symptomatic treatment of Alzheimer's disease (AD). In this study, a series of novel AChE inhibitors were designed and their inhibitory activity was evaluated with 2D
Hachiro Sugimoto et al.
Japanese journal of pharmacology, 89(1), 7-20 (2002-06-27)
A wide range of evidence shows that cholinesterase (ChE) inhibitors can interfere with the progression of Alzheimer's disease (AD). The earliest known ChE inhibitors, namely, physostigmine and tacrine, showed modest improvement in the cognitive function of AD patients. However, clinical
Jong-Il Kim et al.
International journal of pharmaceutics, 455(1-2), 31-39 (2013-08-13)
Although the taste-masking of bitter drug using ion exchange resin has been recognized, in vitro testing using an electronic tongue (e-Tongue) and in vivo bitterness test by human panel test was not fully understood. In case of orally disintegrating tablet
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務