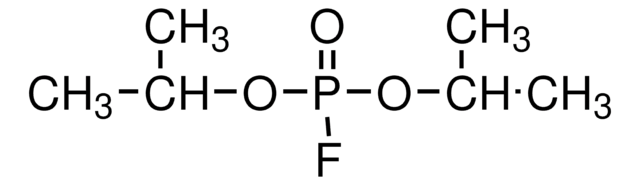推薦產品
應用
四异丙基焦磷酰胺已被用于:
- 作为丁酰胆碱酯酶抑制剂,用于测定猫和虎血浆中丁酰胆碱酯酶 (BChE) 的比例
- 在乙酰胆碱酯酶测定中抑制野生型 BChE
- 选择性阻断 AChE 的酶活性
生化/生理作用
丁酰胆碱酯酶的选择性抑制剂
警告
警告:极其危险!在使用本产品之前,请注意风险并熟悉安全程序。
訊號詞
Danger
危險分類
Acute Tox. 1 Inhalation - Acute Tox. 2 Dermal - Acute Tox. 2 Oral
儲存類別代碼
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
客戶也查看了
Ilaria Corsi et al.
Comparative biochemistry and physiology. Toxicology & pharmacology : CBP, 145(3), 413-419 (2007-02-28)
To address the potential role of cholinesterase enzymes in the invasive capacity of species, the present study investigated ChE activity in the invasive freshwater bivalve Anodonta woodiana (Lea, 1834) comparing it with that of the indigenous species, Anodonta sp. (Linnaeus
Resistance to organophosphorus agent toxicity in transgenic mice expressing the G117H mutant of human butyrylcholinesterase
Wang YW, et al.
Toxicology and Applied Pharmacology, 196(3), 356-366 (2004)
Ming-Kuem Lin et al.
Molecules (Basel, Switzerland), 23(12) (2018-11-24)
The seeds of Cuscuta chinensis Lam. and C. campestris Yuncker have been commonly used as Chinese medical material for preventing aging. Our previous studies have found that C. chinensis and C. campestris possess anti-inflammatory activities in rodents. However, their other
Liyi Geng et al.
PloS one, 8(6), e67446-e67446 (2013-07-11)
Gene transfer of a human cocaine hydrolase (hCocH) derived from butyrylcholinesterase (BChE) by 5 mutations (A199S/F227A/S287G/A328W/Y332G) has shown promise in animal studies for treatment of cocaine addiction. To predict the physiological fate and immunogenicity of this enzyme in humans, a
A A Kousba et al.
Toxicology, 188(2-3), 219-232 (2003-05-28)
The primary mechanism of action for organophosphorus (OP) insecticides such as chlorpyrifos (CPF) involves the inhibition of acetylcholinesterase (AChE) by their active oxon metabolites resulting in a wide range of neurotoxic effects. These oxons also inhibit other cholinesterases (ChE) such
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務