推薦產品
生化/生理作用
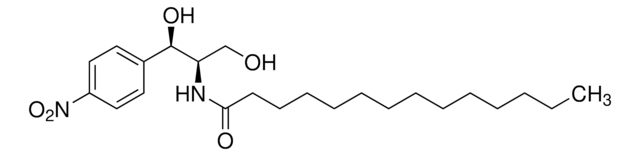
(1R,3S)-HPA-12 is a ceramide (Cer) analog that acts as a Cer transporter (CERT) antagonist and selectively inhibits cellular Cer conversion to sphingomyelin (SM), but not to glucosylceramide, by blocking CERT-mediated Cer ER-to-Golgi transport. Common culture dosing range: 1-10 μM.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Masaharu Ueno et al.
Organic letters, 15(11), 2869-2871 (2013-05-25)
In response to Berkeš's report revising the stereochemistry of HPA-12, an important ceramide-trafficking inhibitor that was discovered and synthesized and its stereochemistry determined in 2001, the synthesis and the stereochemistry were reinvestigated. A large-scale synthetic method for HPA-12 based on
Hannah Scheiblich et al.
Journal of neurochemistry, 143(5), 534-550 (2017-09-25)
Inflammation within the CNS is a major component of many neurodegenerative diseases. A characteristic feature is the generation of microglia-derived factors that play an essential role in the immune response. IL-1β is a pro-inflammatory cytokine released by activated microglia, able
S Yasuda et al.
The Journal of biological chemistry, 276(47), 43994-44002 (2001-09-08)
Ceramide produced at the endoplasmic reticulum (ER) is transported to the lumen of the Golgi apparatus for conversion to sphingomyelin (SM). N-(3-Hydroxy-1-hydroxymethyl-3-phenylpropyl)dodecanamide (HPA-12) is a novel analog of ceramide. Metabolic labeling experiments showed that HPA-12 inhibits conversion of ceramide to
Andrej Ďuriš et al.
Organic letters, 13(7), 1642-1645 (2011-03-01)
The practical stereodivergent route to both syn- and anti-diastereomers of 1-substituted 3-aminobutane-1,4-diols based on the crystallization-induced asymmetric transformation (CIAT) approach was completed. This led to the revision of the reported stereochemistry of the first inhibitor of CERT-dependent ceramide trafficking HPA-12
Yasuhiro Hayashi et al.
The Journal of biological chemistry, 293(45), 17505-17522 (2018-09-23)
Sphingolipids, including sphingomyelin (SM) and glucosylceramide (GlcCer), are generated by the addition of a polar head group to ceramide (Cer). Sphingomyelin synthase 1 (SMS1) and glucosylceramide synthase (GCS) are key enzymes that catalyze the conversion of Cer to SM and
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務