推薦產品
品質等級
化驗
≥98% (HPLC)
形狀
powder
儲存條件
desiccated
顏色
white to tan
溶解度
DMSO: ≥20 mg/mL
儲存溫度
2-8°C
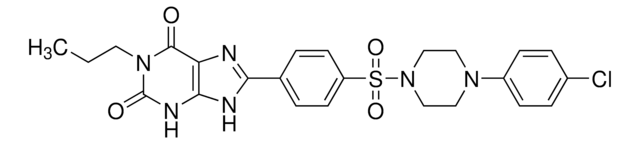
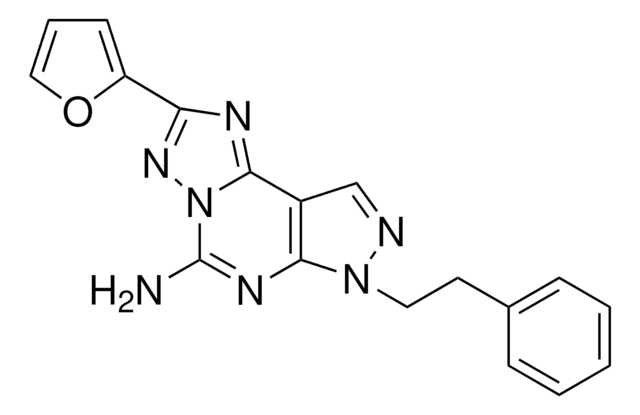
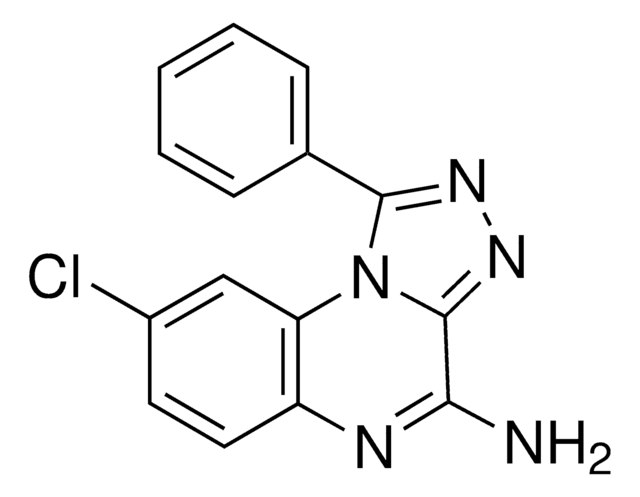
SMILES 字串
CCCCN1C(=O)N(CCCO)c2nc([nH]c2C1=O)C34C[C@@H]5C[C@@H](C[C@H]3C5)C4
InChI
1S/C21H30N4O3/c1-2-3-5-25-18(27)16-17(24(20(25)28)6-4-7-26)23-19(22-16)21-11-13-8-14(12-21)10-15(21)9-13/h13-15,26H,2-12H2,1H3,(H,22,23)/t13-,14+,15-,21-
InChI 密鑰
CIBIXJYFYPFMTN-FZUGUKJMSA-N
應用
PSB36 was used to examine the role of A1-adenosine receptor-mediated cell signaling in CD39 expression in pancreatic b-cells of streptozotocin-induced diabetic mice.
生化/生理作用
Inhibition of A1 adenosine receptor by PSB36 modulates the spinal antinociception in animal models.
PSB36 is a very potent, selective antagonist of the adenosine A1 receptor. The compound selectivity (Ki) for human A1, A2A, A2B and A3 receptors is 0.7, 980, 187 and 2300 respectively. PSB36 is considerably more potent that DPCPX (EC50 0.012 nM vs 2.9 nM)
PSB36 is an adenosine A1 AR antagonist
特點和優勢
This compound is featured on the Adenosine Receptors page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Yuta Tanaka et al.
Biological & pharmaceutical bulletin, 43(3), 516-525 (2019-12-24)
It is therapeutically important to elucidate the factors involved in the radiation resistance of tumors. We previously showed that ATP is released from mouse melanoma B16 cells in response to γ-irradiation, but the role of adenosine, a metabolite of ATP
Osama M Abo-Salem et al.
The Journal of pharmacology and experimental therapeutics, 308(1), 358-366 (2003-10-18)
Caffeine, an adenosine A1, A2A, and A2B receptor antagonist, is frequently used as an adjuvant analgesic in combination with nonsteroidal anti-inflammatory drugs or opioids. In this study, we have examined the effects of novel specific adenosine receptor antagonists in an
Siqi Chen et al.
Cancer immunology research, 8(8), 1064-1074 (2020-05-10)
Accumulating evidence suggests that inhibiting adenosine-generating ecto-enzymes (CD39 and CD73) and/or adenosine A2A or A2B receptors (R) stimulates antitumor immunity and limits tumor progression. Although activating A2ARs or A2BRs causes similar immunosuppressive and protumoral functions, few studies have investigated the
Luca Soattin et al.
Frontiers in physiology, 11, 493-493 (2020-07-01)
Adenosine leads to atrial action potential (AP) shortening through activation of adenosine 1 receptors (A1-R) and subsequent opening of G-protein-coupled inwardly rectifying K+ channels. Extracellular production of adenosine is drastically increased during stress and ischemia. The aim of this study
Kazuki Kitabatake et al.
Biological & pharmaceutical bulletin, 44(2), 197-210 (2020-12-04)
Glioblastoma is the most common malignant tumor of the central nervous system and is treated with a combination of surgery, radiation and chemotherapy. However, the tumor often acquires radiation resistance, which is characterized by an increased DNA damage response (DDR).
文章
We offers many products related to adenosine receptors for your research needs.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務