推薦產品
產品名稱
洛美曲索水合物, ≥95% (HPLC)
化驗
≥95% (HPLC)
形狀
powder
顏色
white to light yellow
溶解度
DMSO: ≥5 mg/mL
儲存溫度
2-8°C
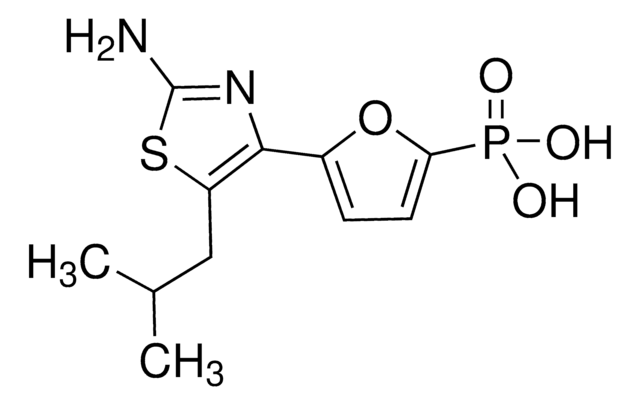
SMILES 字串
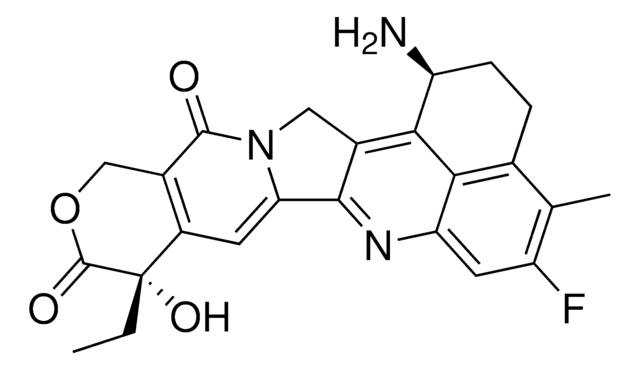
O.NC1=NC(=O)C2=C(NC[C@H](CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)C2)N1
InChI
1S/C21H25N5O6.H2O/c22-21-25-17-14(19(30)26-21)9-12(10-23-17)2-1-11-3-5-13(6-4-11)18(29)24-15(20(31)32)7-8-16(27)28;/h3-6,12,15H,1-2,7-10H2,(H,24,29)(H,27,28)(H,31,32)(H4,22,23,25,26,30);1H2/t12-,15+;/m1./s1
InChI 密鑰
AEFQSKJUVDZANQ-YLCXCWDSSA-N
應用
使用洛美曲索水合物比较人GARFTase有效抑制剂的生物学活性。
生化/生理作用
甘氨酰胺核糖核苷酸甲酰基转移酶(GARFTase)是从头嘌呤合成所需的叶酸依赖性酶。 洛美曲索是GARFTase的有效抑制剂,但不会干扰叶酸合成中涉及的酶。 已在临床上测试了洛美曲索作为一种类似于甲氨蝶呤的抗叶酸药物,用于治疗各种癌症。 用洛美曲索治疗可迅速降低ATP和GTP水平,使细胞周期停滞并诱导细胞凋亡。 尽管核苷酸库的消耗会诱导p53表达,但洛美曲索在野生型和突变型表达p53的肿瘤细胞中均具有细胞毒性。 洛美曲索对CCRF-CEm白血病细胞具有细胞毒性,IC50为2.9 nM。
甘氨酰胺核糖核苷酸甲酰转移酶抑制剂
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Julie L Bronder et al.
Cancer research, 62(18), 5236-5241 (2002-09-18)
The class of folate antimetabolites typified by (6R)-dideazatetrahydrofolate (lometrexol, DDATHF) are specific inhibitors of de novo purine synthesis because of potent inhibition of glycinamide ribonucleotide formyltransferase (GART) but do not induce detectable levels of DNA strand breaks. As such, they
D R Newell
Seminars in oncology, 26(2 Suppl 6), 74-81 (1999-12-22)
Antifolate drugs, as a class, have broad-spectrum activity against both hematologic and solid human malignancies. The pharmacokinetics of the classical antifolate methotrexate have been well-defined and pharmacokinetic data can be exploited to reduce the toxicity and enhance the activity of
Robert Mauritz et al.
Biochemical pharmacology, 63(2), 105-115 (2002-02-14)
We determined the mechanisms of resistance of human CCRF-CEM leukemia cells to methotrexate (MTX) vs. those to six novel antifolates: the polyglutamatable thymidylate synthase (TS) inhibitors ZD1694, multitargeted antifolate, pemetrexed, ALIMTA (MTA) and GW1843U89, the non-polyglutamatable inhibitors of TS, ZD9331
New agents in cancer clinical trials.
J Adams et al.
Oncogene, 19(56), 6687-6692 (2001-06-28)
A Tse et al.
The Journal of biological chemistry, 273(40), 25953-25960 (1998-09-25)
L1210/D3 mouse leukemia cells are resistant to 5, 10-dideazatetrahydrofolate due to expansion of cellular folate pools which block polyglutamation of the drug (Tse, A., and Moran, R. G. (1998) J. Biol. Chem. 273, 25944-25952). These cells were found to have
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務