推薦產品
化驗
≥98% (HPLC)
顏色
yellow to orange
溶解度
DMSO: 5 mg/mL, clear
H2O: insoluble
起源
GlaxoSmithKline
儲存溫度
−20°C
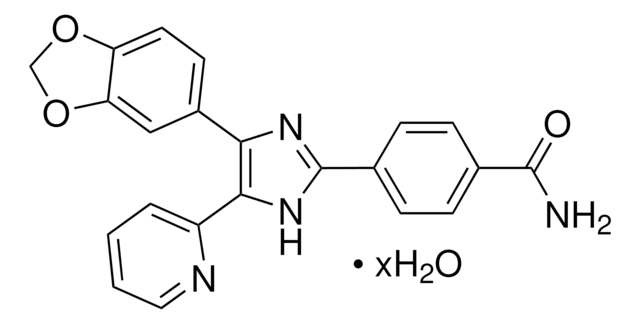
SMILES 字串
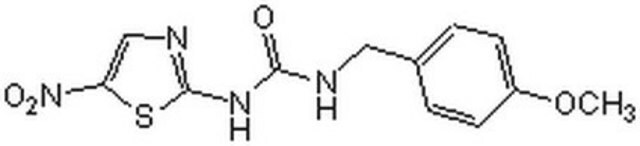
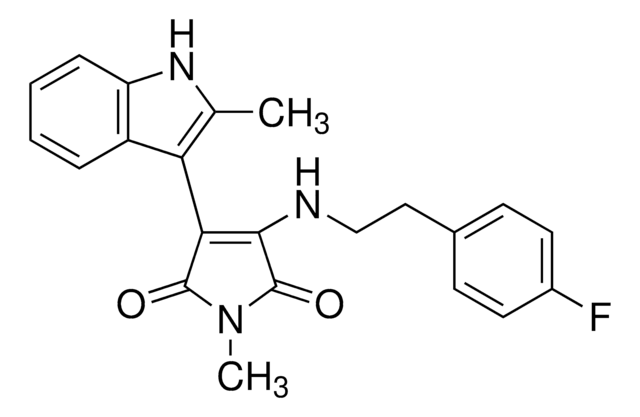
Oc1ccc(NC2=C(C(=O)NC2=O)c3ccccc3[N+]([O-])=O)cc1Cl
InChI
1S/C16H10ClN3O5/c17-10-7-8(5-6-12(10)21)18-14-13(15(22)19-16(14)23)9-3-1-2-4-11(9)20(24)25/h1-7,21H,(H2,18,19,22,23)
InChI 密鑰
PQCXVIPXISBFPN-UHFFFAOYSA-N
基因資訊
human ... GSK3A(2931) , GSK3B(2932)
應用
SB 415286 was used to treat neuroblastoma cells and study the effect of GSK-3 inhibition on cell proliferation.
生化/生理作用
Glycogen synthase kinase-3 (GSK-3) inhibitor.
SB 415286 is a small molecule inhibitor of GSK-3 in muscle and fat cells. SB 415286 induces activation of glycogen synthase and regulates the transport glucose. SB 415286 reduces the systemic inflammation induced by endotoxic shock in rat model of acute colitis. It increases the axonal growth and promotes the recovery of injured adult CNS neurons. SB 415289 is implicated in inducing chromosome instability when used as therapeutic agents.
特點和優勢
This compound is featured on the GSK-3 and PKB/Akt pages of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
This compound was developed by GlaxoSmithKline. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
法律資訊
Sold for research purposes under agreement from GlaxoSmithKline.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
Katrina MacAulay et al.
European journal of biochemistry, 270(18), 3829-3838 (2003-09-03)
Glycogen synthase kinase 3 (GSK3) is inactivated by insulin and lithium and, like insulin, Li also activates glycogen synthase (GS) via inhibition of GSK3. Li also mimics insulin's ability to stimulate glucose transport (GT), an observation that has led to
Daniela Hulcová et al.
Molecules (Basel, Switzerland), 23(4) (2018-03-22)
Glycogen synthase kinase-3β (GSK-3β) is a multifunctional serine/threonine protein kinase that was originally identified as an enzyme involved in the control of glycogen metabolism. It plays a key role in diverse physiological processes including metabolism, the cell cycle, and gene
John Dill et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 28(36), 8914-8928 (2008-09-05)
Axonal regeneration is minimal after CNS injuries in adult mammals and medical treatments to recover neurological deficits caused by axon disconnection are extremely limited. The failure of axonal elongation is principally attributed to the nonpermissive environment and reduced intrinsic growth
Jorge-Tonatiuh Ayala-Sumuano et al.
Scientific reports, 1, 178-178 (2012-02-23)
Adipogenesis is regulated by a complex cascade of transcriptional factors, but little is known about the early events that regulate the adipogenic program. Here, we report the role of the srebf1a gene in the differentiation of fibroblastic 3T3-F442A cells. We
Brendan J R Whittle et al.
British journal of pharmacology, 147(5), 575-582 (2005-11-30)
The effects of the inhibitors of glycogen synthase kinase-3beta (GSK-3beta), TDZD-8 and SB 415286, which can substantially reduce the systemic inflammation associated with endotoxic shock in vivo, have now been investigated on the acute colitis provoked by trinitrobenzene sulphonic acid
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務