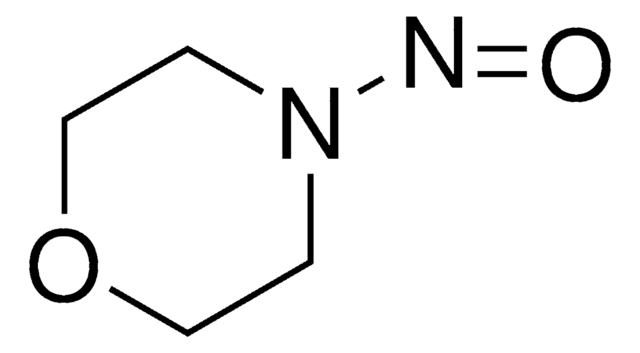
推薦產品
包裝
散装
訊號詞
Danger
危險分類
Acute Tox. 1 Inhalation - Acute Tox. 2 Dermal - Acute Tox. 3 Oral - Carc. 1B
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
K E Appel et al.
Carcinogenesis, 7(4), 659-663 (1986-04-01)
To demonstrate whether there are any pathways of nitrite formation from N-nitrosamines other than reductive denitrosation by cytochrome P-450 we performed the following experiments. An esterified alpha-hydroxylated nitrosamine was incubated in a microsomal system to test if nitrite generation is
J G Farrelly et al.
Cancer research, 42(6), 2105-2109 (1982-06-01)
With the use of rat liver preparations, the in vitro microsomal metabolism of methylethylnitrosamine, methyl-n-butylnitrosamine, and methyl(2-phenylethyl)nitrosamine labeled with deuterium in the methyl and alpha-methylene positions has been compared with that of the parent (unlabeled) compounds. All three forms of
M Lee et al.
Cancer research, 49(6), 1470-1474 (1989-03-15)
Metabolic activation may be a key step in determining the tissue specificity of carcinogenic nitrosamines. In previous work, we characterized P450IIE1 (an acetone/ethanol-inducible form of cytochrome P-450) as the major enzyme for the metabolic activation of N-nitrosodimethylamine. In this work
T Gichner et al.
Mutagenesis, 1(2), 107-109 (1986-03-01)
The organic solvents dimethylsulphoxide (DMSO), acetone, ethanol and dimethylformamide inhibited the mutagenic activity of the promutagens dimethylnitrosamine and methylbutylnitrosamine in a higher plant Arabidopsis thaliana. In contrast, the direct-acting mutagens N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) and N-methyl-N-nitrosourea (MNU) were not affected by the
Q Huang et al.
Cancer letters, 69(2), 107-116 (1993-04-30)
We studied the metabolism of methyl-n-butyl-nitrosamine (MBN), a carcinogen for the rat esophagus and liver. The 2-, 3- and 4-hydroxy derivatives were identified as new metabolites of MBN. In studies on tissue slices freshly removed from MRC-Wistar rats, MBN metabolism
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務