推薦產品
生物源
synthetic (organic)
品質等級
化驗
≥95%
形狀
powder or flakes
solid
溶解度
DMSO: 4 mg/mL
儲存溫度
−20°C
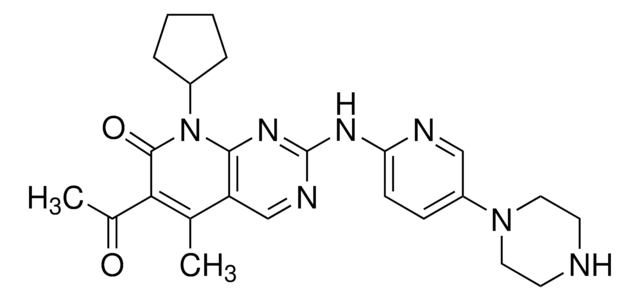
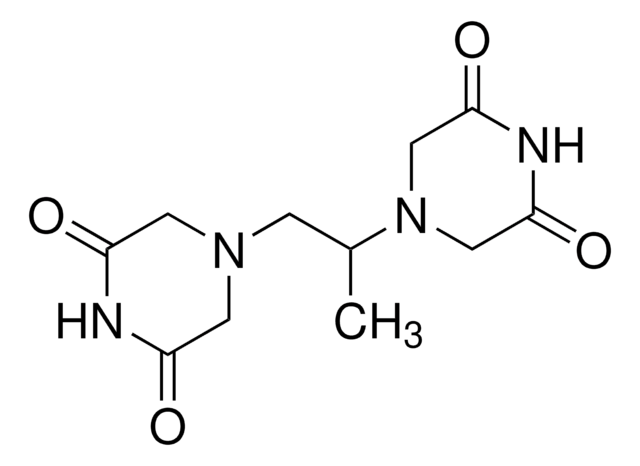
SMILES 字串
C[C@@H]([C@@H](C)N1CC(=O)NC(=O)C1)N2CC(=O)NC(=O)C2
InChI
1S/C12H18N4O4/c1-7(15-3-9(17)13-10(18)4-15)8(2)16-5-11(19)14-12(20)6-16/h7-8H,3-6H2,1-2H3,(H,13,17,18)(H,14,19,20)/t7-,8+
InChI 密鑰
OBYGAPWKTPDTAS-OCAPTIKFSA-N
一般說明
ICRF-193是一种双二哌嗪衍生物。它通过形成不可裂解的复合物来抑制拓扑异构酶II。
應用
ICRF-193已被用作拓扑异构酶II(TOP2)抑制剂,用于处理小鼠卵母细胞,以研究TOP2在减数分裂中的作用。
生化/生理作用
ICRF-193有助于增强细胞周期而不会导致无染色体分离。 它被认为是急性早幼粒细胞白血病(APL)的重要化学分化治疗药物。ICRF-193可用作抗癌药之间的分化诱导剂。
ICRF-193诱导一个G2检查点,该检查点与ATR依赖性的polo样激酶1(plk1)活性抑制和细胞周期蛋白B1磷酸化水平降低有关。 诱导K562和Molt-4等多种细胞系的凋亡。 ICRF-193是拓扑异构酶II抑制剂,对拓扑异构酶II-β比拓扑异构酶II-α更有效,可能还会导致DNA链断裂。
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Skin Sens. 1
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
ICRF-193, a catalytic inhibitor of DNA topoisomerase II, delays the cell cycle progression from metaphase, but not from anaphase to the G1 phase in mammalian cells
Iwai M, et al.
Febs Letters, 406(3), 267-270 (1997)
N Hajji et al.
Mutation research, 530(1-2), 35-46 (2003-10-18)
The bis-dioxopiperazine ICRF-193 has long time been considered as a pure topoisomerase II catalytic inhibitor able to exert its inhibitory effect on the enzyme without stabilization of the so-called cleavable complex formed by DNA covalently bound to topoisomerase II. In
The catalytic DNA topoisomerase II inhibitor ICRF-193 and all-trans retinoic acid cooperatively induce granulocytic differentiation of acute promyelocytic leukemia cells: candidate drugs for chemo-differentiation therapy against acute promyelocytic leukemia
Niitsu,
Experimental Hematology, 30(11), 1273-1282 (2002)
K C Huang et al.
The Journal of biological chemistry, 276(48), 44488-44494 (2001-09-29)
Antineoplastic bis(dioxopiperazine)s, such as meso-2,3-bis(2,6-dioxopiperazin-4-yl)butane (ICRF-193), are widely believed to be only catalytic inhibitors of topoisomerase II. However, topoisomerase inhibitors have little or no antineoplastic activity unless they are topoisomerase poisons, a special subclass of topoisomerase-targeting drugs that stabilize topoisomerase-DNA
Sílvia Dyson et al.
The EMBO journal, 40(1), e105393-e105393 (2020-11-07)
The juxtaposition of intracellular DNA segments, together with the DNA-passage activity of topoisomerase II, leads to the formation of DNA knots and interlinks, which jeopardize chromatin structure and gene expression. Recent studies in budding yeast have shown that some mechanism
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務