推薦產品
溶解度
alcohol: soluble
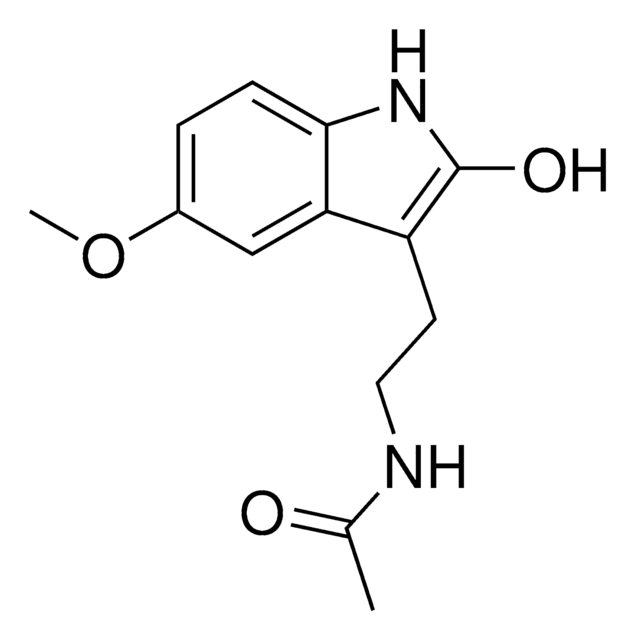
SMILES 字串
COc1cc2c(CCNC(C)=O)c[nH]c2cc1O
InChI
1S/C13H16N2O3/c1-8(16)14-4-3-9-7-15-11-6-12(17)13(18-2)5-10(9)11/h5-7,15,17H,3-4H2,1-2H3,(H,14,16)
InChI 密鑰
OMYMRCXOJJZYKE-UHFFFAOYSA-N
基因資訊
human ... MTNR1A(4543) , MTNR1B(4544)
尋找類似的產品? 前往 產品比較指南
一般說明
6-Hydroxymelatonin (6-OHM) is a melatonin metabolite. It is produced in the liver by the action of cytochrome P450 enzyme as well as by photodegradation of melatonin. In the central nervous system, it exists as a sulfated form. 6-OHM is a partial melatonin receptor MT2 agonist.
應用
6-Hydroxymelatonin has been used as a melatonin derivative to test its protective effects in ultra violet B (UVB)-induced oxidative stress melanocytes and keratinocytes.
生化/生理作用
6-Hydroxymelatonin (6-OHM) is an antioxidant with a free radical scavenging role. Like melatonin, it reduces the impact of UVB-induced oxidative stress in melanocytes. It also aids protection during iron (Fe2+)-induced neurotoxicity. 6-OHM effectively reduces lipid peroxidation and superoxide anion production induced by potassium cyanide (KCN).
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Carc. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Deepa S Maharaj et al.
Journal of neurochemistry, 96(1), 78-81 (2005-11-23)
Oxidative damage of biological macromolecules is a hallmark of most neurodegenerative disorders such as Alzheimer, Parkinson and diffuse Lewy body diseases. Another important phenomenon involved in these disorders is the alteration of iron homeostasis, with an increase in iron levels.
Xuwan Liu et al.
American journal of physiology. Heart and circulatory physiology, 283(1), H254-H263 (2002-06-14)
The present study was designed to explore the protective effects of melatonin and its analogs, 6-hydroxymelatonin and 8-methoxy-2-propionamidotetralin, on the survival of doxorubicin-treated mice and on doxorubicin-induced cardiac dysfunction, ultrastructural alterations, and apoptosis in mouse hearts. Whereas 60% of the
Katsuhisa Sakano et al.
Biochemical pharmacology, 68(9), 1869-1878 (2004-09-29)
Melatonin, an indolic pineal hormone, is produced primarily at night in mammals and is important in controlling biological rhythms. Although melatonin is known to be effective as a free radical scavenger and has an anti-cancer effect, carcinogenic properties have also
G Facciolá et al.
European journal of clinical pharmacology, 56(12), 881-888 (2001-04-25)
The present study was carried out to identify the cytochrome P450 enzyme(s) involved in the 6-hydroxylation and O-demethylation of melatonin. The formation kinetics of 6-hydroxymelatonin and N-acetylserotonin were determined using human liver microsomes and cDNA yeast-expressed human enzymes (CYP1A2, 2C9
S Härtter et al.
Therapeutic drug monitoring, 23(3), 282-286 (2001-05-22)
Melatonin has recently garnered interest as a possible treatment for sleep disorders, and this has created a desire for appropriate pharmacokinetic studies. No method has yet been published that can measure the concentrations of both melatonin and its main metabolite
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務