G115060
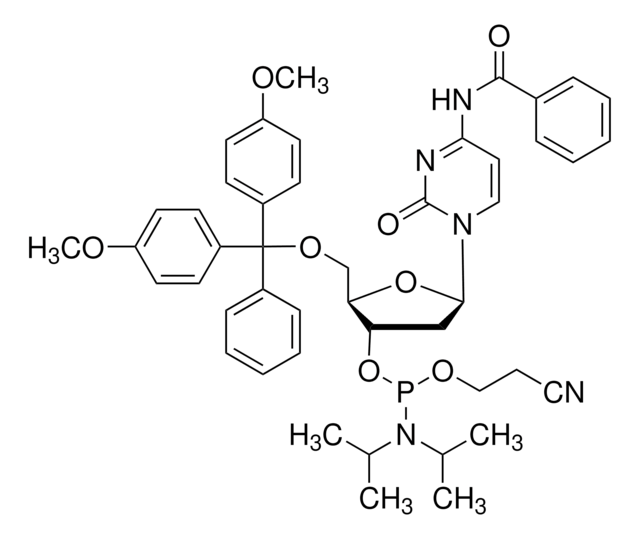
DMT-dG(dmf) Phosphoramidite
configured for ABI
同義詞:
DMT-dG(dmf) Amidite, N-[(二甲基氨基)亚甲基]-5′-O- [双(4-甲氧基苯基)苯基甲基]-2′-脱氧鸟苷,3′-[2-氰基乙基N,N-双(1-甲基乙基)亚磷酰胺], N2-二甲基甲脒基-5′-O-(4,4′-二甲氧基三苯甲基)-2′-脱氧鸟苷-3′-O-[O-(2-氰基乙基)-N,N′-二异丙基亚]
About This Item
推薦產品
種類
for DNA synthesis
品質等級
產品線
Proligo Reagents
化驗
≥99% (31P-NMR)
≥99.0% (reversed phase HPLC)
形狀
powder
技術
oligo synthesis: suitable
顏色
white to off-white
&lambda ;
conforms (UV/VIS Identity)
相容性
configured for ABI
核苷譜
base: deoxyguanosine
base protecting group: DMF
2' protecting group: none
5' protecting group: DMT
deprotection: fast/standard
儲存溫度
-10 to -25°C
SMILES 字串
COc1ccc(cc1)C(OC[C@H]2O[C@H](C[C@@H]2OP(OCCC#N)N(C(C)C)C(C)C)n3cnc4C(=O)NC(\N=C/N(C)C)=Nc34)(c5ccccc5)c6ccc(OC)cc6
InChI
1S/C43H53N8O7P/c1-29(2)51(30(3)4)59(56-24-12-23-44)58-36-25-38(50-28-45-39-40(50)47-42(48-41(39)52)46-27-49(5)6)57-37(36)26-55-43(31-13-10-9-11-14-31,32-15-19-34(53-7)20-16-32)33-17-21-35(54-8)22-18-33/h9-11,13-22,27-30,36-38H,12,24-26H2,1-8H3,(H,47,48,52)/b46-27-/t36-,37+,38+,59?/m0/s1
InChI 密鑰
YRQAXTCBMPFGAN-UJASEYITSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
- dG(dmf) is deprotected faster than the conventional dG(ib): thedeprotection time in concentrated ammonia is reduced to 2 hours at55 °C or 1 hour at 65 °C
- The dG(dmf)-monomer is especially suitable for G-rich sequences:incomplete deprotection is greatly reduced in comparison with theconventional dG(ib)-monomer
- dG(dmf)-phosphoramidite is as stable in solution as the standard dA(bz)-,dC(bz)- and dT-phosphoramidites
- dG(dmf)-phosphoramidite can directly substitute for dG(ib)-phosphoramidite
- No change is required in the reagents commonly used for DNA synthesis(except a low concentration iodine oxidizer i.e., 0.02 M in iodine, should beemployed)DMT-dG(dmf) Phosphoramidite is configured for ABI Synthesizers."
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務