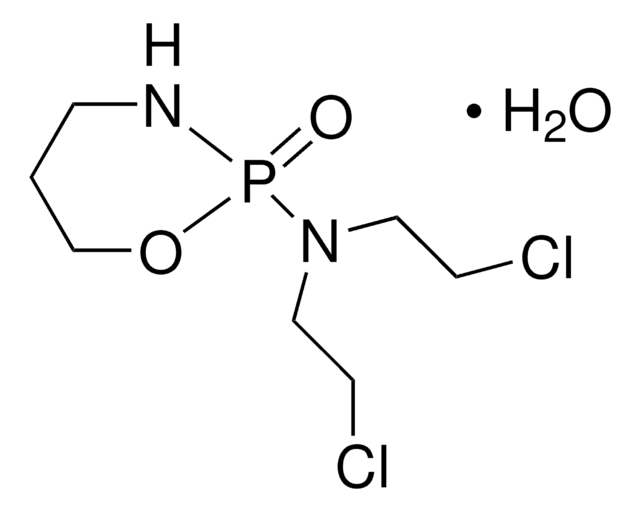
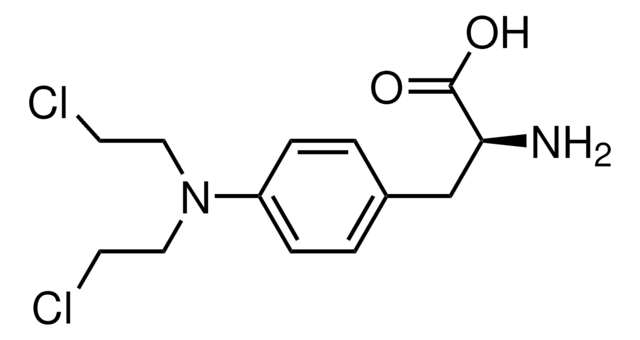
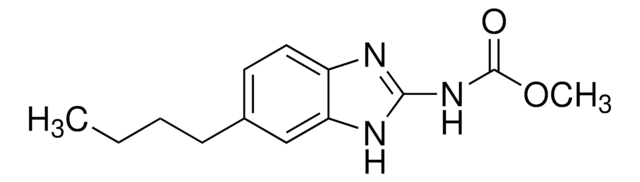
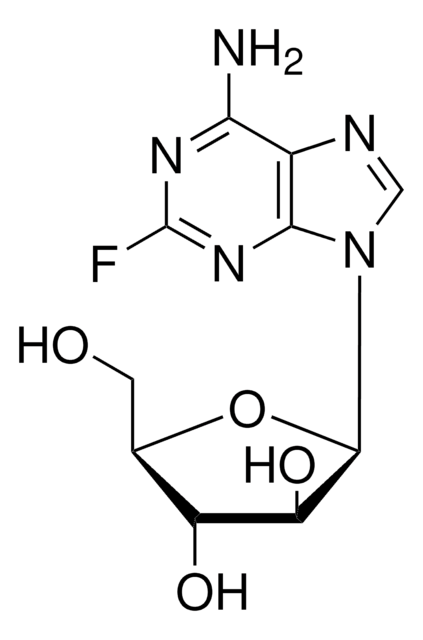
推薦產品
形狀
powder
品質等級
顏色
white
溶解度
DMSO: soluble
抗生素活性譜
neoplastics
作用方式
DNA synthesis | interferes
儲存溫度
−20°C
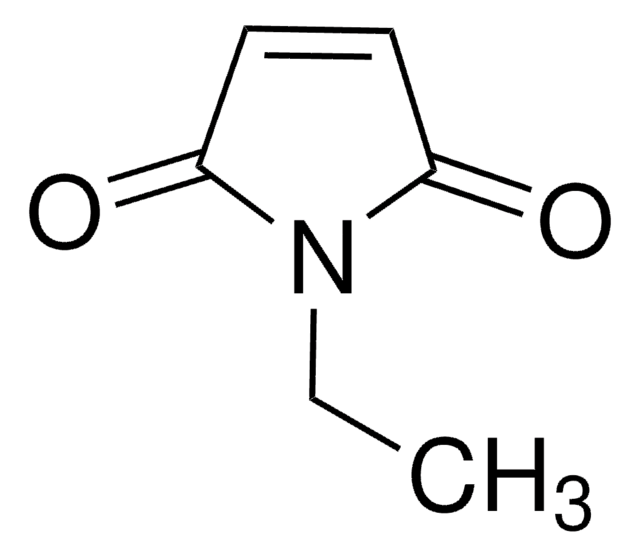
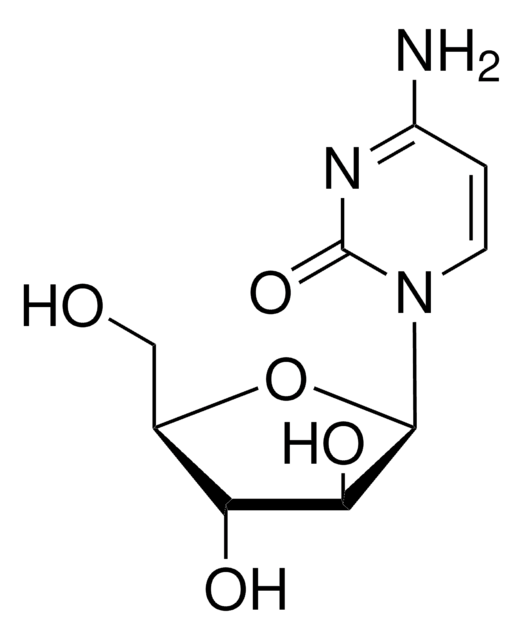
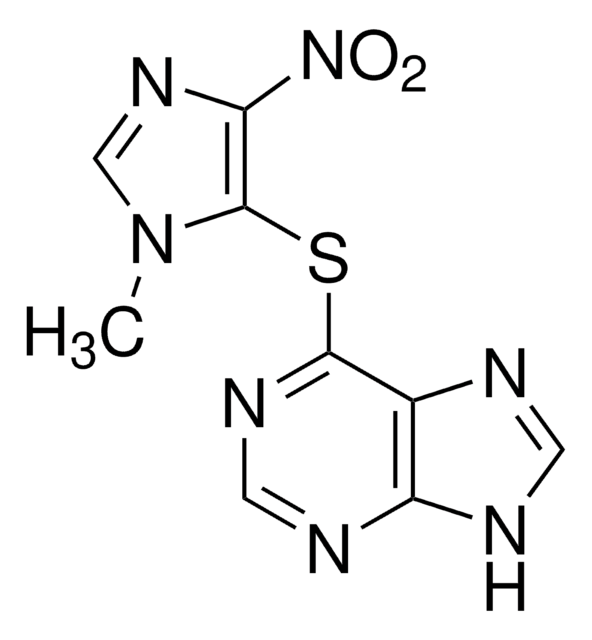
SMILES 字串
Fc1nc2[n](cnc2c(n1)[N+H3])C3OC(C(C3O)O)CO[P](=O)([O-])O
InChI
1S/C10H13FN5O7P/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(18)5(17)3(23-9)1-22-24(19,20)21/h2-3,5-6,9,17-18H,1H2,(H2,12,14,15)(H2,19,20,21)
InChI 密鑰
GIUYCYHIANZCFB-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Fludarabine is a purine analog included in the category of DNA-damaging drugs with well-known efficacy in B-cell chronic lymphocytic leukemia (B-CLL).
應用
- Characterization of Chemical Interactions between Clinical Drugs and the Oral Bacterium, Corynebacterium matruchotii, via Bioactivity-HiTES.: This study explores the interactions of clinical drugs like Fludarabine phosphate with Corynebacterium matruchotii, highlighting potential impacts on oral microbiota and implications for drug efficacy and safety (Lee DY et al., 2024).
- Cocktail of lipophilic and hydrophilic chemotherapeutics in high-load core@shell nanocarriers to treat pancreatic tumours.: Investigates the efficacy of a combination of Fludarabine phosphate with other chemotherapeutics delivered via nanocarriers, aiming to enhance treatment outcomes for pancreatic cancer by improving drug delivery to the tumor site (Rudolph D et al., 2024).
- Macrophage neogenin deficiency exacerbates myocardial remodeling and inflammation after acute myocardial infarction through JAK1-STAT1 signaling.: This research demonstrates the role of Fludarabine phosphate in modulating inflammation and cardiac repair post-myocardial infarction, offering insights into its potential therapeutic benefits beyond oncology (Zhang J et al., 2023).
- SLC25A51 promotes tumor growth through sustaining mitochondria acetylation homeostasis and proline biogenesis.: Discusses the cellular mechanisms by which Fludarabine phosphate may influence metabolic pathways in cancer cells, highlighting its potential to disrupt tumor metabolism and promote cancer cell death (Li Y et al., 2023).
- CD19-Targeting CAR T Cells for Myositis and Interstitial Lung Disease Associated With Antisynthetase Syndrome.: Reviews the use of Fludarabine phosphate in preconditioning regimens for CAR T-cell therapy, emphasizing its role in enhancing the efficacy of immunotherapy in treating autoimmune disorders (Pecher AC et al., 2023).
生化/生理作用
Fludarabine represses DNA replication and suppresses the nucleotide metabolism by inhibiting the enzyme ribonucleotide reductase.
訊號詞
Warning
危險聲明
危險分類
Muta. 2 - Repr. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Francesca Ricci et al.
Therapeutics and clinical risk management, 5(1), 187-207 (2009-05-14)
Fludarabine (FAMP) is the most effective and most extensively studied purine analog in indolent B-cell malignancies. Its use is indicated for first-and second-line treatment of B-cell chronic lymphocytic leukemia (B-CLL). FAMP as a single agent has produced superior response rates
Andrea Celeghin et al.
Cell death & disease, 7(12), e2562-e2562 (2016-12-30)
Besides its canonical role in stabilizing telomeres, telomerase reverse transcriptase (TERT) may promote tumorigenesis through extra-telomeric functions. The possible therapeutic effects of BIBR1532 (BIBR), a powerful TERT inhibitor, have been evaluated in different cellular backgrounds, but no data are currently
Míriam Molina-Arcas et al.
Blood, 101(6), 2328-2334 (2002-11-02)
Nucleoside derivatives are currently used in the treatment of hematologic malignancies. Although intracellular events involved in the pharmacologic action of these compounds have been extensively studied, the role of plasma membrane transporters in nucleoside-derived drug bioavailability and action in leukemia
W Plunkett et al.
Seminars in oncology, 17(5 Suppl 8), 3-17 (1990-10-01)
Fludara I.V. (fludarabine phosphate) (9-beta-D-arabinosyl-2-fluoroadenine, F-ara-A) is an adenine nucleoside analogue resistant to adenosine deaminase that shows promising therapeutic activity in the clinical treatment of lymphocytic hematologic malignancies. F-ara-A is transported into cells, where it is converted to its 5'-triphosphate
H M Kantarjian et al.
Seminars in oncology, 17(5 Suppl 8), 66-70 (1990-10-01)
The promising results obtained with Fludara I.V. (fludarabine phosphate) treatment in the common indolent B-cell neoplasms have led to their further evaluation in other unusual B-cell malignancies, in Hodgkin's disease, and in T-cell diseases. A significant response rate has been
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務