About This Item
推薦產品
品質等級
化驗
≥98% (HPLC)
形狀
solid
光學活性
[α]/D +14 to +19°, c = 1 in chloroform-d
儲存條件
desiccated
顏色
white
溶解度
H2O: soluble ≥20 mg/mL
起源
Eli Lilly
儲存溫度
room temp
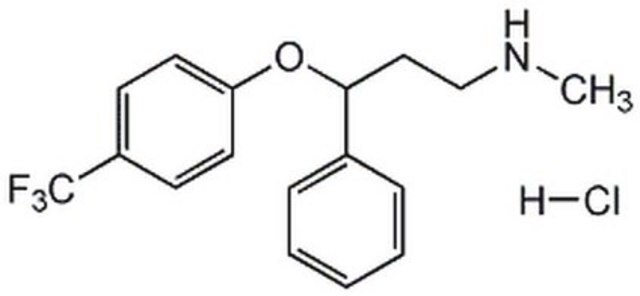
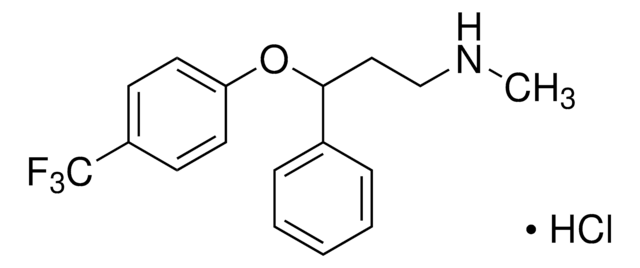
SMILES 字串
Cl.CNCC[C@H](Oc1ccc(cc1)C(F)(F)F)c2ccccc2
InChI
1S/C17H18F3NO.ClH/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20;/h2-10,16,21H,11-12H2,1H3;1H/t16-;/m0./s1
InChI 密鑰
GIYXAJPCNFJEHY-NTISSMGPSA-N
基因資訊
human ... HTR1A(3350) , HTR1B(3351) , HTR1D(3352) , HTR1E(3354) , HTR1F(3355) , HTR2A(3356) , HTR2B(3357) , HTR2C(3358) , HTR3A(3359) , HTR3B(9177) , HTR3C(170572) , HTR3D(200909) , HTR3E(285242) , HTR4(3360) , HTR5A(3361) , HTR5B(645694) , HTR6(3362) , HTR7(3363)
一般說明
生化/生理作用
特點和優勢
法律資訊
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Chromatograms
suitable for GC我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務