推薦產品
化驗
≥98% (HPLC)
形狀
powder
顏色
white to off-white
溶解度
DMSO: >20 mg/mL
儲存溫度
2-8°C
SMILES 字串
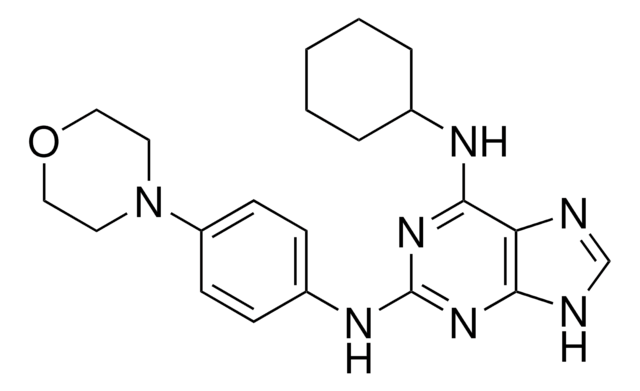
CC1=C(C(NC(=O)N1CCCCCC(O)=O)c2ccc(cc2)-c3ccccc3)C(=O)OCc4ccccc4
InChI
1S/C31H32N2O5/c1-22-28(30(36)38-21-23-11-5-2-6-12-23)29(32-31(37)33(22)20-10-4-9-15-27(34)35)26-18-16-25(17-19-26)24-13-7-3-8-14-24/h2-3,5-8,11-14,16-19,29H,4,9-10,15,20-21H2,1H3,(H,32,37)(H,34,35)
InChI 密鑰
GHFQWLNXJMUCGC-UHFFFAOYSA-N
應用
116-9e has been used as an inhibitor of heat shock protein 70 (Hsp70) co-chaperone HDJ2 (human Ydj1/DNAJA1) to study the influence of HDJ2 on the regulation of ribonucleotide reductase (RNR) activity in HEK293 cells. It has also been used as an Hsp70 inhibitor to study the effect of Hsp70 chaperone on b5‐ops glycosylation in the side populations (SP) HeLa cells.
生化/生理作用
116-9e is a blocker of Hsp40-Hsp70 binding thereby inhibiting the chaperone activity of Hsp70-Hsp40. The Hsp40 family of co–chaperones binds to Hsp70 through a conserved J–domain. It is believed that 116-9e inhibits chaperone functions by preventing Hsp70–Hsp40 complex assembly.
The same compound by a different name, MAL2-11B, has been found to inhibit the activity of a viral J-domain protein, large tumor antigen (TAg). MAL2-11B inhibited both TAg′s endogenous ATPase activity and the TAg-mediated activation of Hsp70.
The same compound by a different name, MAL2-11B, has been found to inhibit the activity of a viral J-domain protein, large tumor antigen (TAg). MAL2-11B inhibited both TAg′s endogenous ATPase activity and the TAg-mediated activation of Hsp70.
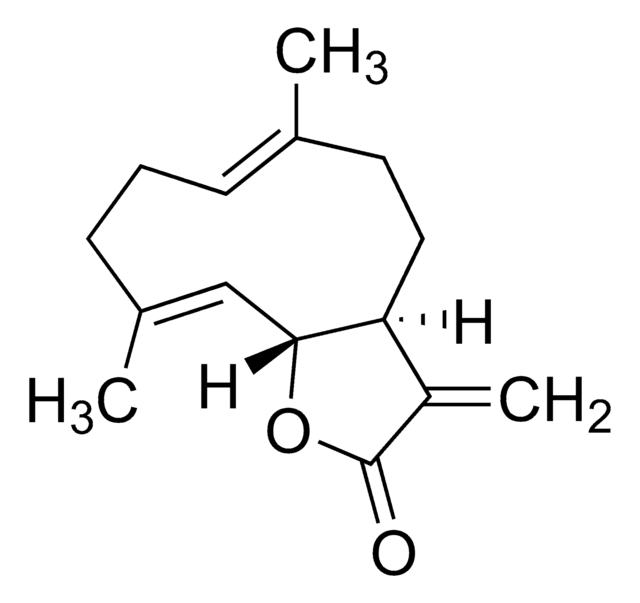
116-9e is a dihhydropyrimidine compound.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1
儲存類別代碼
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Bruna Figueiredo Costa et al.
Traffic (Copenhagen, Denmark), 19(3), 182-197 (2018-01-24)
Tail-anchored (TA) proteins insert into their target organelles by incompletely elucidated posttranslational pathways. Some TA proteins spontaneously insert into protein-free liposomes, yet target a specific organelle in vivo. Two spontaneously inserting cytochrome b5 forms, b5-ER and b5-RR, which differ only
Erina Matsuoka et al.
Functional plant biology : FPB (2019-06-21)
The heat shock protein 90 (HSP90) inhibitor, geldanamycin, is a chemical inducer of the heat shock response (HSR) in Arabidopsis. Geldanamycin is thought to activate the heat shock signal by dissociating the HSP90-heat shock factor (HSF) complex. Recent studies have
Isaac T Sluder et al.
PLoS genetics, 14(11), e1007462-e1007462 (2018-11-20)
Hsp70 is a well-conserved molecular chaperone involved in the folding, stabilization, and eventual degradation of many "client" proteins. Hsp70 is regulated by a suite of co-chaperone molecules that assist in Hsp70-client interaction and stimulate the intrinsic ATPase activity of Hsp70.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務