全部照片(4)
About This Item
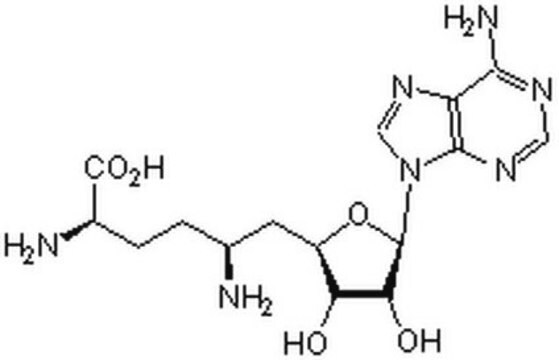
經驗公式(希爾表示法):
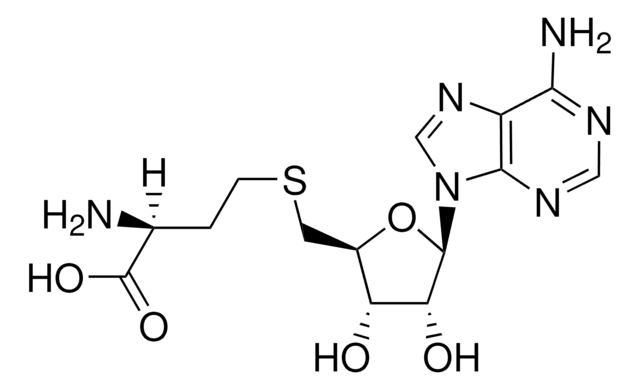
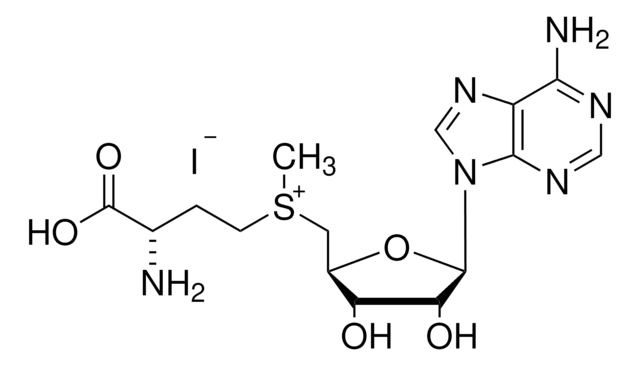
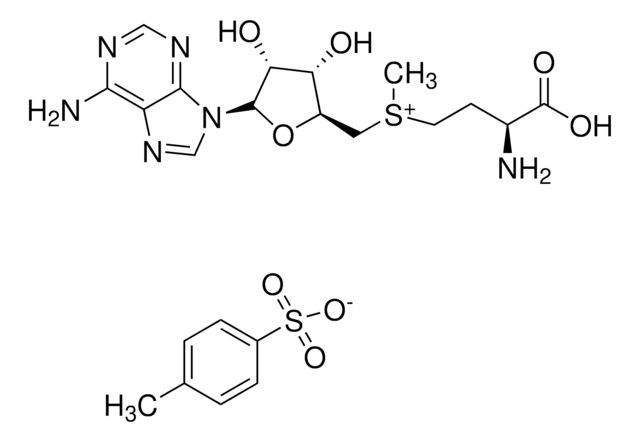
C15H23ClN6O5S · 2 HCl
CAS號碼:
分子量::
507.82
Beilstein:
6560002
MDL號碼:
分類程式碼代碼:
12352209
PubChem物質ID:
NACRES:
NA.32
推薦產品
生物源
synthetic (chemical)
品質等級
化驗
≥75%
形狀
powder
顏色
white to off-white
溶解度
H2O: 100 mg/mL
應用
cell analysis
運輸包裝
dry ice
儲存溫度
−20°C
SMILES 字串
Cl.Cl.[Cl-].C[S+](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n2cnc3c(N)ncnc23
InChI
1S/C15H22N6O5S.3ClH/c1-27(3-2-7(16)15(24)25)4-8-10(22)11(23)14(26-8)21-6-20-9-12(17)18-5-19-13(9)21;;;/h5-8,10-11,14,22-23H,2-4,16H2,1H3,(H2-,17,18,19,24,25);3*1H/t7-,8+,10+,11+,14+,27?;;;/m0.../s1
InChI 密鑰
KBAFOJZCBYWKPU-XQVUROGGSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
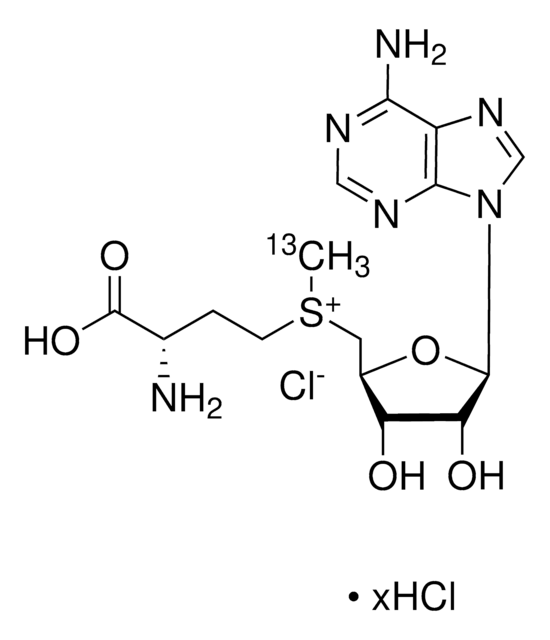
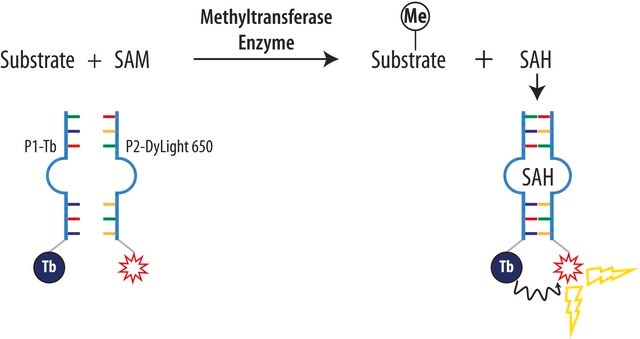
S-(5′-腺苷)-L-蛋氨酸氯化二盐酸盐(SAM)有助于将甲基基团转移到蛋白质,脂类和核酸上。蛋氨酸腺苷基转移酶催化从蛋氨酸和腺苷三磷酸(ATP)合成SAM。SAM能够调节各种细胞功能,比如细胞分裂,细胞死亡,翻译,遗传稳定性,氧化剂/抗氧化剂平衡和聚胺平衡。从治疗上来说,SAM可用作人体营养补充剂,用于多种疾病比如骨关节炎和肝脏损伤。SAM还能调节转硫化反应,通过和关键酶硫醚-β-合成酶(CBS)调控域结合实现。
應用
S-(5′-腺苷)-L-甲硫氨酸氯化二盐酸盐(SAM)被用作S30细胞提取物培养基补充剂。通过石英晶体微平衡(QCM),表面等离子共振(SPR)和等温滴定量热(ITC),它可以用来测量苯邻二酚-O-甲基转移酶(COMT)和支撑脂双层以及载体互作。
生化/生理作用
甲基供体;甲基酶催化辅因子,包括儿茶酚O-甲基转移酶(COMT)和DNA甲基转移酶(DNMT)。
甲基供体;甲基酶催化辅因子,包括儿茶酚O-甲基转移酶(COMT)和DNA甲基转移酶(DNMT)。 尽管存在于所有细胞中,它主要集中在肝脏中,这里是85%甲基化反应发生的地方。 它同样参与了调节肝脏功能,生长和伤害应答。
注意
这种材料在制备是是80%-90%纯,但是非常不稳定。已经有记录每天在25℃能够损伤10%纯度。
分析報告
纯度基于UV和HPLC。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Leah C Beauchamp et al.
Journal of Alzheimer's disease : JAD, 77(4), 1705-1715 (2020-09-15)
Alterations in the methionine cycle and abnormal tau phosphorylation are implicated in many neurodegenerative diseases, including Alzheimer's disease and frontotemporal dementia. rTg4510 mice express mutant human P301L tau and are a model of tau hyperphosphorylation. The cognitive deficit seen in
Chemical communication between bacteria and cell-free gene expression systems within linear chains of emulsion droplets
Schwarz-Schilling M, et al.
Integrative Biology : Quantitative Biosciences from Nano to Macro, 8(4), 564-570 (2016)
Yan Zhang et al.
Archives of microbiology, 191(10), 773-783 (2009-09-05)
Enolase-phosphatase (E1), as an enzyme, is involved in methionine salvage pathway in many prokaryotic and eukaryotic organisms. But the identity and function of E1 in Xanthomonas oryzae pv. oryzae (Xoo) remain undetermined. Here, we report the cloning and characterization of
Andria V Rodrigues et al.
Chembiochem : a European journal of chemical biology, 21(5), 663-671 (2019-09-13)
We recently reported the discovery of phenylacetate decarboxylase (PhdB), representing one of only ten glycyl-radical-enzyme reaction types known, and a promising biotechnological tool for first-time biochemical synthesis of toluene from renewable resources. Here, we used experimental and computational data to
Ted M Lakowski et al.
Analytical biochemistry, 396(1), 158-160 (2009-09-08)
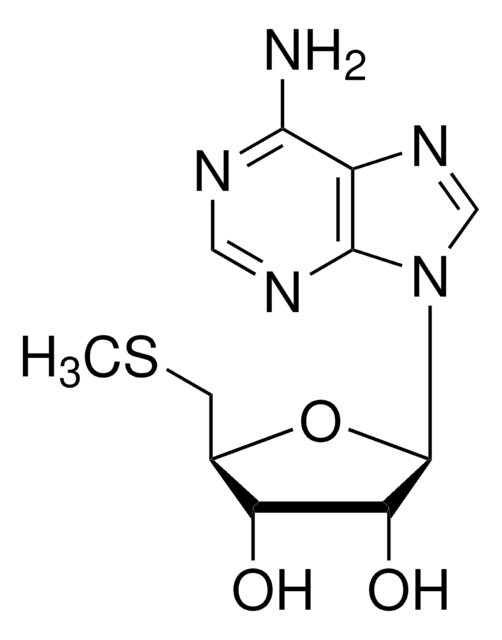
S-Adenosyl-L-homocysteine (AdoHcy) background signal in reactions with protein arginine N-methyltransferase 1 is investigated using an ultrahigh-performance liquid chromatography tandem mass spectrometry assay that measures AdoHcy. We identify three sources of AdoHcy background: enzymatic automethylation, AdoHcy contamination in commercial S-adenosyl-L-methionine (AdoMet)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務