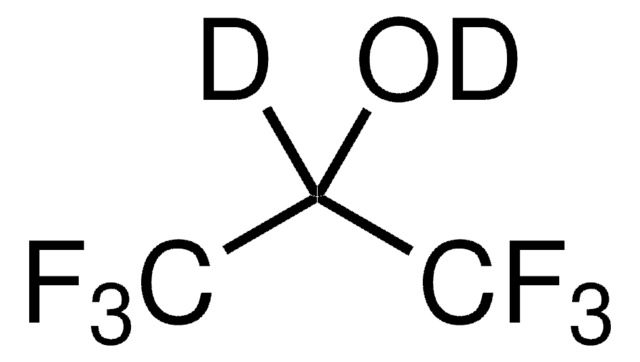
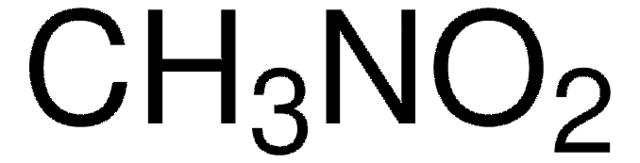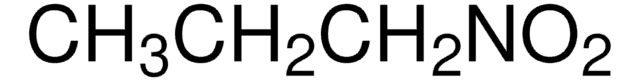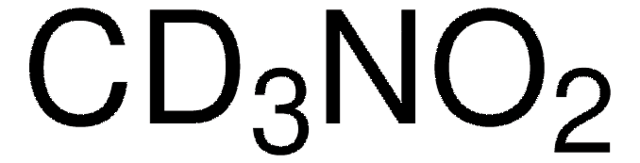推薦產品
等級
reagent
蒸汽密度
2.1 (vs air)
蒸汽壓力
2.7 mmHg
產品線
ReagentPlus®
化驗
≥99.0%
形狀
liquid
自燃溫度
784 °F
expl. lim.
7.3 %, 33 °F
dilution
(for general lab use)
折射率
n20/D 1.382 (lit.)
pH值
6.4 (20 °C, 0.01 g/L)
bp
101.2 °C (lit.)
mp
−29 °C (lit.)
密度
1.127 g/mL at 25 °C (lit.)
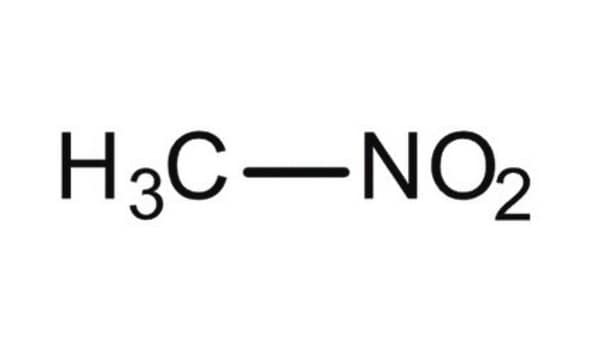
SMILES 字串
C[N+]([O-])=O
InChI
1S/CH3NO2/c1-2(3)4/h1H3
InChI 密鑰
LYGJENNIWJXYER-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Nitromethane (CH3NO2) is the simplest nitro organic compound used for a wide range of applications, including as polar solvents to racing fuel. It serves as a solvent for organic chemistry and as a valuable building block for various useful compounds like nitroalkanes, β-nitroalcohols, nitroalkenes, carbonyl compounds, amines, and heterocycles. In industry, nitromethane can be used to stabilize halogenated hydrocarbons.
應用
Nitromethane can be used:
- As a reagent in the synthesis of 3-nitroindoles by arylation with N-(2-bromoaryl) imidates using palladium catalyst.
- As a cyanating reagent for the synthesis of thiocyanates in presence of base (KOAc) and iodine.
- In the preparation of cobalt cage complexes from polyamines and formaldehyde.
- As a solvent in the preparation of β-substituted γ-nitroaldehydes by enantioselective cross-coupling of β-arylated or γ,δ-unsaturated aldehydes using oxidizing agent DDQ (2,3-dichloro-5,6-dicyanoquinone) and catalyst diphenylprolinol silyl ether.
特點和優勢
Nitromethane as high energy monopropellant exhibits
- Low toxicity.
- High specific impulse.
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Warning
危險分類
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Flam. Liq. 3 - Repr. 2
儲存類別代碼
4.1A - Other explosive hazardous materials
水污染物質分類(WGK)
WGK 2
閃點(°F)
95.0 °F - closed cup
閃點(°C)
35 °C - closed cup
客戶也查看了
The catalytic chemistry of nitromethane over Co-ZSM5 and other catalysts in connection with the methane-NOxSCR reaction.
Cowan AD, et al.
J. Catal., 176(2), 329-343 (1998)
Metal ion encapsulation: cobalt cages derived from polyamines, formaldehyde, and nitromethane.
Geue RJ, et al.
Journal of the American Chemical Society, 106(19), 5478-5488 (1984)
Benedek Vakulya et al.
Organic letters, 7(10), 1967-1969 (2005-05-07)
Cinchona alkaloid-derived chiral bifunctional thiourea organocatalysts were synthesized and applied in the Michael addition between nitromethane and chalcones with high ee and chemical yields.
Atanu Bhattacharya et al.
The Journal of chemical physics, 136(2), 024321-024321 (2012-01-21)
Decomposition of electronically excited nitro-containing molecules with different X-NO(2) (X = C, N, O) moieties has been intensively investigated over the past decades; however, their decomposition behavior has not previously been compared and contrasted. Comparison of their unimolecular decomposition behavior
Eagleson M.
Concise Encyclopedia Chemistry, 696-696 (1994)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務