全部照片(4)
About This Item
線性公式:
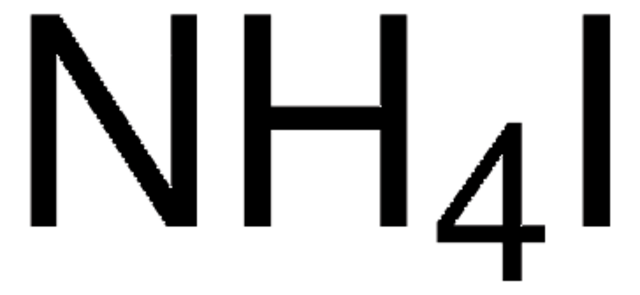
NH4I
CAS號碼:
分子量::
144.94
EC號碼:
MDL號碼:
分類程式碼代碼:
12352300
eCl@ss:
38050607
PubChem物質ID:
推薦產品
等級
ACS reagent
蒸汽壓力
1 mmHg ( 210.9 °C)
化驗
≥99%
形狀
powder or crystals
環保替代產品特色
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
雜質
≤0.005% insolubles
燃燒殘留物
≤0.05%
pH值
4.5-6.5 (25 °C, 50 g/L)
負離子痕跡
Cl- and Br-: ≤0.005%
phosphate (PO43-): ≤0.001%
sulfate (SO42-): ≤0.05%
正離子痕跡
Ba: ≤0.002%
Fe: ≤5 ppm
heavy metals (as Pb): ≤0.001%
環保替代類別
SMILES 字串
N.I
InChI
1S/HI.H3N/h1H;1H3
InChI 密鑰
UKFWSNCTAHXBQN-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
我们致力于为您带来符合一项或多项绿色化学十二原则的环保替代产品。本品可用作合成许多杂环化合物的原料,也可用作某些反应的催化剂或氧化还原催化剂。点击此处以获取更多信息。
使用以碘化铵为催化剂的二氧化碳将未活化的氮丙啶高收率和区域选择性转化为恶唑烷酮
使用以碘化铵为催化剂的二氧化碳将未活化的氮丙啶高收率和区域选择性转化为恶唑烷酮
訊號詞
Danger
危險聲明
危險分類
STOT RE 1 Oral
標靶器官
Thyroid
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Jinho Kim et al.
Organic letters, 14(15), 3924-3927 (2012-07-17)
Copper-mediated regioselective cyanation of indoles and 2-phenylpyridines was developed by using ammonium iodide and DMF as the combined source of a cyano unit under "Pd-free" conditions. Mechanistic studies indicate that the reaction of indoles proceeds through a two-step sequence: electrophilic
Xiaofang Gao et al.
Chemical communications (Cambridge, England), 51(1), 210-212 (2014-11-20)
A novel ammonium iodide-induced sulfonylation of alkenes with DMSO and water toward the synthesis of vinyl methyl sulfones is described. The process proceeded smoothly under metal-free conditions with high stereoselectivity and good functional group tolerance. The reaction mechanism was revealed
Simple and regioselective oxyiodination of aromatic compounds with ammonium iodide and Oxone.
Mohan KVVK, et al.
Tetrahedron Letters, 45(43), 8015-8018 (2004)
Low-Frequency Vibrations in Ammonium Iodide and Ammonium Bromide.
Durig JR and Antion DJ.
J. Chem. Phys. , 51(9), 3639-3647 (1969)
Jinho Kim et al.
Journal of the American Chemical Society, 134(5), 2528-2531 (2012-01-28)
The cyanation of aromatic boronic acids, boronate esters, and borate salts was developed under copper-mediated oxidative conditions using ammonium iodide and DMF as the source of nitrogen and carbon atom of the cyano unit, respectively. The procedure was successfully extended
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務