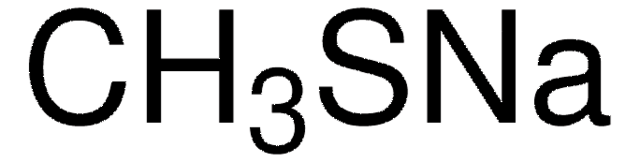推薦產品
等級
ACS reagent
蒸汽密度
2.67 (vs air)
蒸汽壓力
5.83 psi
化驗
≥99.9%
形狀
liquid
自燃溫度
212 °F
expl. lim.
50 %
dilution
(for analytical testing)
雜質
H2S, passes test (lim. ~1.5 ppm)
SO2, passes test (lim ~2.5 ppm)
≤0.05% water
蒸發殘留物
≤0.002%
顏色
APHA: ≤10
折射率
n20/D 1.627 (lit.)
bp
46 °C (lit.)
mp
−112-−111 °C (lit.)
密度
1.266 g/mL at 25 °C (lit.)
SMILES 字串
S=C=S
InChI
1S/CS2/c2-1-3
InChI 密鑰
QGJOPFRUJISHPQ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Carbon disulfide (CS2) is a colorless liquid. It is present in atmosphere and natural waters in the form of sulfur gas. It is responsible for the production of sulfur dioxide (SO2) in the atmosphere (stratosphere and/or troposphere). Its lifetime in the atmosphere has been proposed to be around 12 days. It is a non-polar solvent.
Carbon disulfide is a highly volatile, flammable liquid with low ignition temperature. It can be synthesized by reacting hydrocarbon gas with sulfur. It is an important raw material for preparing viscose rayon and cellophane film.
應用
Carbon disulfide has been used for the desorption of air samples during analysis of trihalomethanes (THMs) in those samples.
It may be used in the preparation of dibenzyl trithiocarbonate, a RAFT (Reversible Addition-Fragmentation Transfer) chain-transfer agent (CTA). It may be employed as a solvent in the preparation of benzyl chlorodithioformate.
It may be used in the preparation of dibenzyl trithiocarbonate, a RAFT (Reversible Addition-Fragmentation Transfer) chain-transfer agent (CTA). It may be employed as a solvent in the preparation of benzyl chlorodithioformate.
Carbon disulfide may be used in the xanthogenation of cellulose and in the synthesis of poly(ethylene trithiocarbonate) and dithiocarbamates. C2S may undergo hydrolysis catalyzed by nanosized titania and zirconia to form CO2 and H2S. It can also generate 1,3-dithiolium carbenes via cycloaddition with acetylenes.
包裝
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險分類
Acute Tox. 4 Inhalation - Eye Irrit. 2 - Flam. Liq. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1
標靶器官
Peripheral nervous system,Central nervous system,Cardio-vascular system,Eyes
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
-22.0 °F - closed cup
閃點(°C)
-30 °C - closed cup
Copolymerization of ethylene sulfide and carbon disulfide.
Soga K, et al.
Journal of Polymer Science, 14(3), 677-684 (1976)
Carbon Disulfide.
Smith DE and Timmerman RW.
Kirk-Othmer Encyclopedia of Chemical Technology (2003)
An efficient, one-pot, synthesis of dithiocarbamates from the corresponding alcohols using Mitsunobu's reagent.
Chaturvedi D and Ray S.
Tetrahedron Letters, 47(8), 1307-1309 (2006)
Cyril Catto et al.
International journal of environmental research and public health, 9(8), 2562-2586 (2012-10-16)
In order to improve disinfection by-product (DBP) exposure assessment, this study was designed to document both water and air levels of these chemical contaminants in two indoor swimming pools and to analyze their within-day and day-to-day variations in both of
Nanosized titania and zirconia as catalysts for hydrolysis of carbon disulfide.
Yue Y, et al.
Applied Catalysis. B, Environmental, 46(3), 561-572 (2003)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務