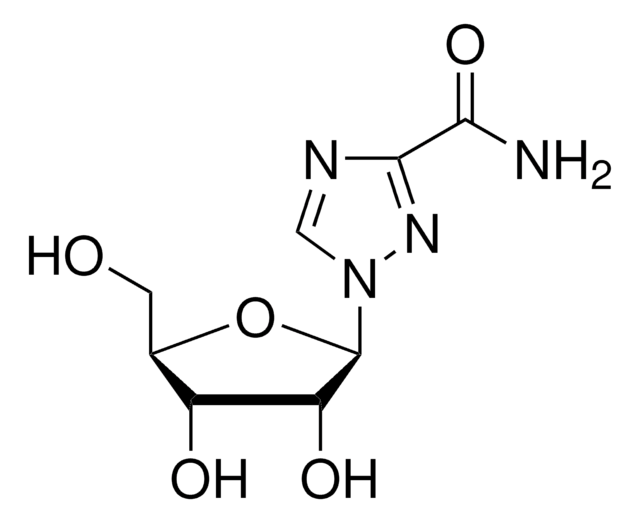
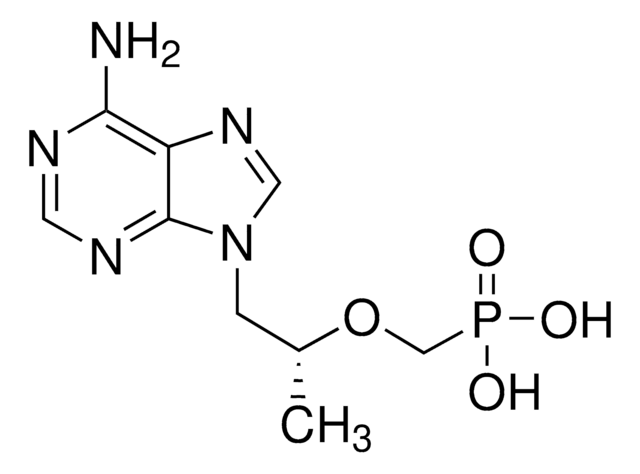
推薦產品
等級
pharmaceutical primary standard
API 家族
zidovudine
製造商/商標名
EDQM
mp
113-115 °C (lit.)
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
CC1=CN([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO)O2)C(=O)NC1=O
InChI
1S/C10H13N5O4/c1-5-3-15(10(18)12-9(5)17)8-2-6(13-14-11)7(4-16)19-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,18)/t6-,7+,8+/m0/s1
InChI 密鑰
HBOMLICNUCNMMY-XLPZGREQSA-N
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Zidovudine EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
对 HIV-1 病毒有效的逆转录酶抑制剂。
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Carc. 2 - Muta. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
R Bendayan et al.
Pharmacotherapy, 15(3), 338-344 (1995-05-01)
In humans and various animal species, 3'-azido-3'-deoxythymidine (AZT) is in part eliminated by the kidneys, where it undergoes significant tubular secretion. The goal of this project was to develop, in a continuous renal epithelial cell line (LLCPK1), a model of
Tomas Cihlar et al.
Antiviral research, 85(1), 39-58 (2009-11-06)
Twenty-five years ago, nucleoside analog 3'-azidothymidine (AZT) was shown to efficiently block the replication of HIV in cell culture. Subsequent studies demonstrated that AZT acts via the selective inhibition of HIV reverse transcriptase (RT) by its triphosphate metabolite. These discoveries
Cátia Teixeira et al.
European journal of medicinal chemistry, 46(4), 979-992 (2011-02-25)
The first anti-HIV drug, zidovudine (AZT), was approved by the FDA a quarter of a century ago, in 1985. Currently, anti-HIV drug-combination therapies only target HIV-1 protease and reverse transcriptase. Unfortunately, most of these molecules present numerous shortcomings such as
D M Simpson
Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 29(1), 19-34 (1999-08-05)
Human immunodeficiency virus (HIV)-associated dementia (HIVD) has been reported in up to 15% of HIV-infected adult patients. Although the pathogenesis of HIVD remains unclear, HIV probably plays an important role in the syndrome, as evidenced by the correlation between cerebrospinal
A combination drug of abacavir-lamivudine-zidovudine (Trizivir) for treating HIV infection and AIDS.
Muki Shey et al.
The Cochrane database of systematic reviews, (3)(3), CD005481-CD005481 (2009-07-10)
The human immunodeficiency virus (HIV) has become one of the greatest challenges to global public health. In 2007 UNAIDS estimated that 33.2 million people were living with HIV. Currently recommended regimens for initiating HIV treatment consist of either a non-nucleoside
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務