推薦產品
等級
pharmaceutical primary standard
API 家族
gestodene
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
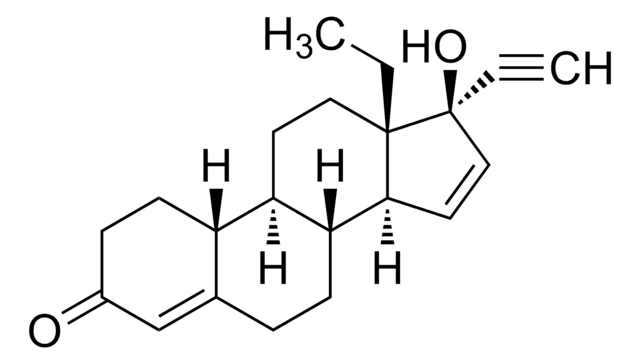
CC[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@H]34)[C@@H]1C=C[C@@]2(O)C#C
InChI
1S/C21H26O2/c1-3-20-11-9-17-16-8-6-15(22)13-14(16)5-7-18(17)19(20)10-12-21(20,23)4-2/h2,10,12-13,16-19,23H,3,5-9,11H2,1H3/t16-,17+,18+,19-,20-,21-/m0/s1
InChI 密鑰
SIGSPDASOTUPFS-XUDSTZEESA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Gestodene EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
Gestodene is a synthetic progestin used as a contraceptive.
Gestodene is a synthetic progestin used as a contraceptive. Gestodene displays a high binding affinity to the progesterone receptor, and also binds strongly to adrogen and glucocorticoid receptors.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Repr. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
Ladakan Jaithitivit et al.
Journal of the Medical Association of Thailand = Chotmaihet thangphaet, 95(5), 630-635 (2012-09-22)
To determine cycle control, safety, and acceptability of a 24-day oral contraceptive regimen containing 15 micrograms of ethinylestradiol and 60 micrograms of gestodene. This was an open-label, non-comparative study. Healthy women 18 to 35 years old who attended the Family
Romana Dmitrovic et al.
Obstetrics and gynecology, 119(6), 1143-1150 (2012-05-24)
To estimate whether continuous oral contraceptive pills (OCPs) will result in more pain relief in primary dysmenorrhea patients than cyclic OCPs, which induce withdrawal bleeding with associated pain and symptoms. We conducted a double-blind, randomized, controlled trial comparing continuous to
Sopon Cheewadhanaraks et al.
Gynecologic and obstetric investigation, 74(2), 151-156 (2012-06-23)
To evaluate the efficacy and tolerability of postoperative depot medroxyprogesterone acetate (DMPA) versus postoperative continuous oral contraceptive (OC) pills in the treatment of endometriosis-associated pain. After a conservative surgery, 84 patients with symptomatic endometriosis were randomized to receive either intramuscular
Øjvind Lidegaard et al.
BMJ (Clinical research ed.), 343, d6423-d6423 (2011-10-27)
To assess the risk of venous thromboembolism from use of combined oral contraceptives according to progestogen type and oestrogen dose. National historical registry based cohort study. Four registries in Denmark. Non-pregnant Danish women aged 15-49 with no history of thrombotic
Hormone-based contraceptive therapy and risk of venous thromboembolism in young women.
Helen Roberts
Clinical advances in hematology & oncology : H&O, 8(5), 307-309 (2010-06-17)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務