推薦產品
品質等級
化驗
≥98% (HPLC)
形狀
powder
光學活性
[α]/D +80 to +95°, c = 1 (CHCl3)
顏色
white to beige
溶解度
DMSO: >5 mg/mL
儲存溫度
−20°C
SMILES 字串
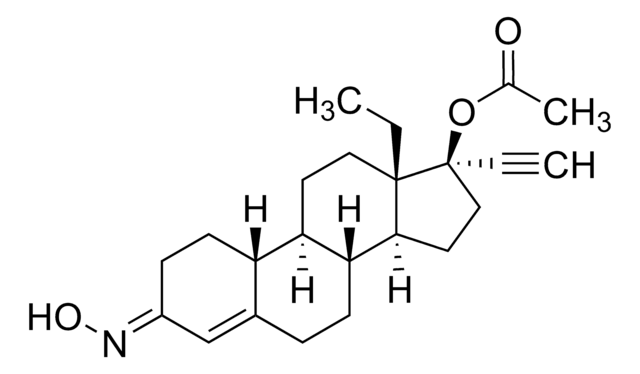
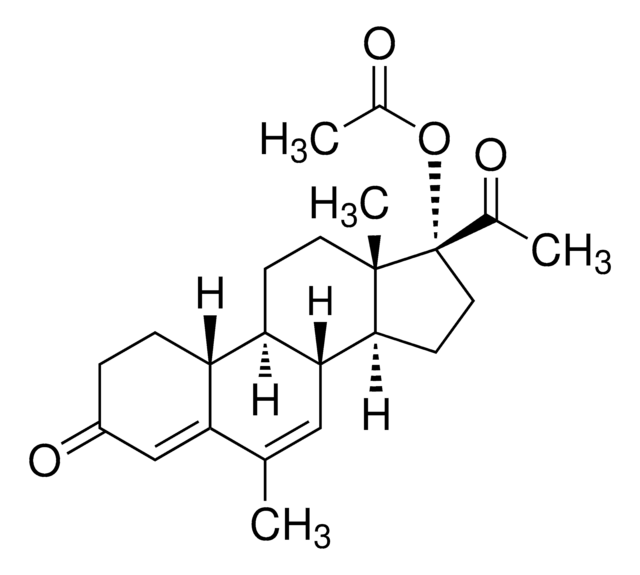
CC[C@]12CC(=C)[C@H]3[C@@H](CCC4=CC(=O)CC[C@H]34)[C@@H]1CC[C@@]2(O)C#C
InChI
1S/C22H28O2/c1-4-21-13-14(3)20-17-9-7-16(23)12-15(17)6-8-18(20)19(21)10-11-22(21,24)5-2/h2,12,17-20,24H,3-4,6-11,13H2,1H3/t17-,18-,19-,20+,21-,22-/m0/s1
InChI 密鑰
GCKFUYQCUCGESZ-BPIQYHPVSA-N
基因資訊
human ... PGR(5241)
尋找類似的產品? 前往 產品比較指南
生化/生理作用
Etonogestrel is a lipophilic molecule. Pharmacokinetics studies reveal that etonogestrel is bound to protein albumin in the blood. This remains independent of both endogenous and exogenous concentration of estradiol.
Etonogestrel is a third-generation progestin contraceptive. Etonogestrel is used as a contraceptive itself and is also the active metabolite of the drug desogestrel used in some combination contraceptives.
特點和優勢
This compound is featured on the Nuclear Receptors (Steroids) page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
訊號詞
Danger
危險聲明
危險分類
Carc. 2 - Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Pharmacokinetics of etonogestrel released from the contraceptive implant Implanon
Wenzl R, et al.
Contraception, 58(5), 283-288 (1998)
Peter Schnabel et al.
Clinical drug investigation, 32(6), 413-422 (2012-05-01)
The etonogestrel (ENG)-releasing implant is a subdermal progestogen-only contraceptive that provides coverage for up to 3 years. This long-acting hormonal contraceptive has been available in Europe since 1998 and in the US since 2006. To date, localization of non-palpable implants
Kristina M Tocce et al.
American journal of obstetrics and gynecology, 206(6), 481-481 (2012-05-29)
The purpose of this study was to determine contraceptive continuation and repeat pregnancy rates in adolescents who are offered immediate postpartum etonogestrel implant (IPI) insertion. Participants in an adolescent prenatal-postnatal program were enrolled in a prospective observational study of IPI
Diana Mansour et al.
Contraception, 83(3), 202-210 (2011-02-12)
The aim of this guidance is to review the management of unacceptable vaginal bleeding patterns in etonogestrel (ENG)-releasing contraceptive implant users concentrating, where possible, on the evidence for pharmacological treatments and identifying a pragmatic approach where this is not possible.
Anne Calhoun et al.
Headache, 52(8), 1246-1253 (2012-07-14)
To determine whether extended-cycle dosing of an ultralow dose vaginal ring contraceptive decreases frequency of migraine aura and prevents menstrual related migraine (MRM). Many women are denied therapy with combined hormonal contraceptives due to published guidelines that recommend against their
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務