推薦產品
生物源
synthetic
等級
pharmaceutical primary standard
agency
EP
API 家族
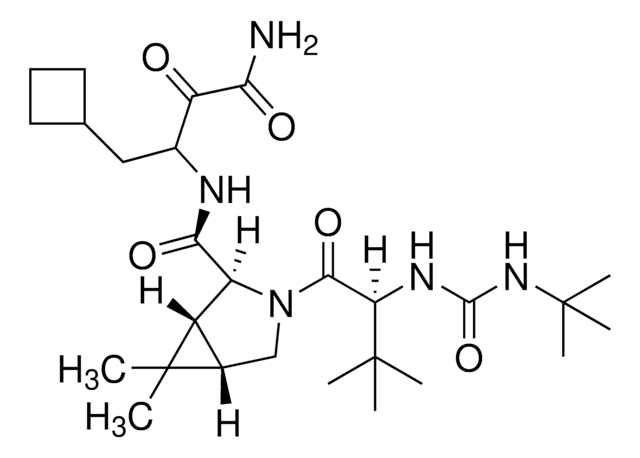
ritonavir
包裝
pkg of 80 mg
製造商/商標名
EDQM
儲存條件
protect from light
溶解度
ethanol: soluble
methanol: soluble
應用
pharmaceutical (small molecule)
形式
neat
運輸包裝
ambient
儲存溫度
2-8°C
InChI
1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1
InChI 密鑰
NCDNCNXCDXHOMX-XGKFQTDJSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Ritonavir belongs to the group of protease inhibitors that are widely used in combination with other drugs in the prevention of HIV. Its mode of action involves binding to the active site of the protease enzyme and preventing the further maturation of new viral particles.
Ritonavir belongs to the group of protease inhibitors that are widely used in combination with other drugs in the prevention of HIV. Its mode of action involves binding to the active site of the protease enzyme and preventing the further maturation of new viral particles.
應用
This European Pharmacopoeia reference standard is intended for use only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Alicia Khululwa Khwitshana et al.
South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde, 104(7), 495-500 (2014-09-13)
HIV research is a therapeutic area for which well-defined population-specific treatment and prophylaxis guidelines exist. However, there are limited objective, evidence-based data for assessing adherence to these guidelines. To conduct a retrospective, cross sectional study of adult HIV-infected patients receiving
Mónica M Calderón et al.
Pharmacotherapy, 34(11), 1151-1158 (2014-08-22)
Panax ginseng has been shown in preclinical studies to modulate cytochrome P450 enzymes involved in the metabolism of HIV protease inhibitors. Therefore, the purpose of this study was to determine the influence of P. ginseng on the pharmacokinetics of the
RP-HPLC method development and validation for simultaneous estimation of Lopinavir and Ritonavir in tablet dosage form
Bindu MJ, et al.
International Journal of Science and Engineering Applications, 5(1), 77-77 (2017)
HPTLC method for simultaneous determination of lopinavir and ritonavir in capsule dosage form
Sulebhavikar VA, et al.
Journal of Chemistry, 5(4), 706-712 (2008)
J J M A Hendrikx et al.
British journal of cancer, 110(11), 2669-2676 (2014-05-02)
The intestinal uptake of the taxanes paclitaxel and docetaxel is seriously hampered by drug efflux through P-glycoprotein (P-gp) and drug metabolism via cytochrome P450 (CYP) 3A. The resulting low oral bioavailability can be boosted by co-administration of P-gp or CYP3A4
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務