推薦產品
等級
pharmaceutical primary standard
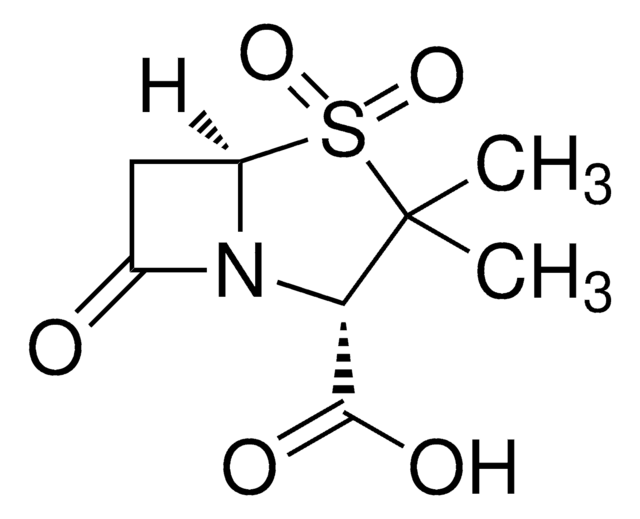
API 家族
sulbactam
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
InChI
1S/C8H11NO5S.Na/c1-8(2)6(7(11)12)9-4(10)3-5(9)15(8,13)14;/h5-6H,3H2,1-2H3,(H,11,12);/q;+1/p-1/t5-,6+;/m1./s1
InChI 密鑰
NKZMPZCWBSWAOX-IBTYICNHSA-M
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Sulbactam sodium EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
Maura S de Oliveira et al.
Clinics (Sao Paulo, Brazil), 68(4), 569-573 (2013-06-20)
The objective of this study was to evaluate whether the outcomes of carbapenem-resistant Acinetobacter infections treated with ampicillin/sulbactam were associated with the in vitro susceptibility profiles. Twenty-two infections were treated with ampicillin/sulbactam. The median treatment duration was 14 days (range:
Y-T Lee et al.
European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology, 32(9), 1211-1220 (2013-04-05)
Tigecycline (TG) has been shown to be active in vitro against Acinetobacter baumannii, although data on the clinical efficacy of TG alone or in combination for the treatment of infections due to multidrug-resistant A. baumannii (MDRAB) remain limited. The purpose
Alaa A Hassan et al.
Archiv der Pharmazie, 346(7), 562-570 (2013-06-19)
(E)-4-Aryl-2-[2-(1-substituted ethylidene)hydrazinyl]thiazoles and (Z)-3-substituted-4-aryl-2-[(E)-(1-phenylethylidene)hydrazono]-2,3-dihydrothiazoles were synthesized by the reaction of (substituted ethylidene)hydrazinecarbothioamides with ω-bromoacetophenones. The characterization of this new class of compounds was performed using different spectroscopic tools. The structure of (Z)-3-benzyl-4-(4-bromophenyl)-2-[(E)-(1-phenylethylidene)hydrazono]-2,3-dihydrothiazole 6e was unambiguously confirmed by single-crystal X-ray crystallography.
Seyedali Seyedmajidi et al.
Arab journal of gastroenterology : the official publication of the Pan-Arab Association of Gastroenterology, 14(1), 1-5 (2013-04-30)
Selection of the best drug regimens for eradication of Helicobacter pylori infection especially in patients at risk of peptic ulcer relapses and the development of complications is challenging. This study assessed and compared the efficacy of the two common PPI
Mengtao Zhou et al.
Pancreatology : official journal of the International Association of Pancreatology (IAP) ... [et al.], 13(3), 212-215 (2013-05-31)
Our aim was to investigate the efficiency of continuous regional intra-arterial infusion (CRAI) with antisecretory agents and antibiotics in the treatment of infected pancreatic necrosis. CRAI was used as a new clinical technique to treat acute pancreatitis patients during a
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務