推薦產品
等級
pharmaceutical primary standard
API 家族
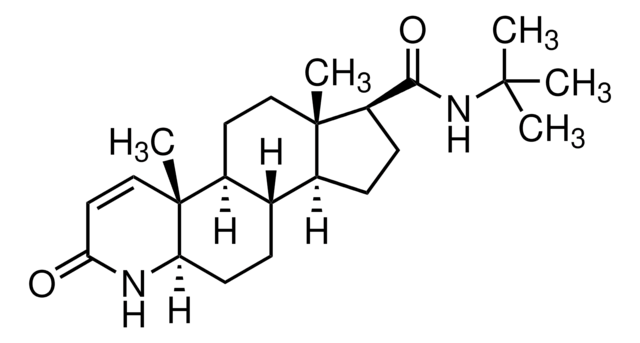
finasteride
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
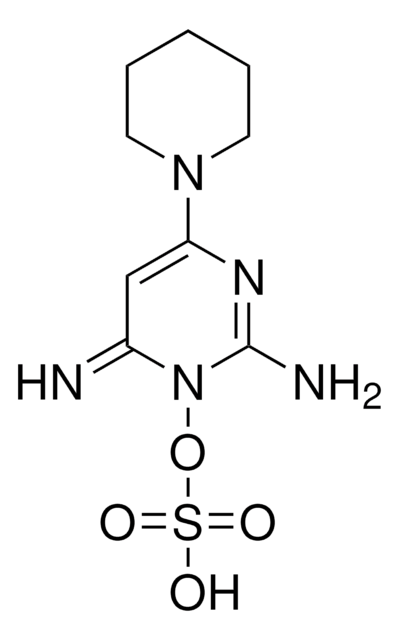
SMILES 字串
[H][C@@]12CC[C@@]3([H])[C@]4([H])CC[C@H](C(=O)NC(C)(C)C)[C@@]4(C)CC[C@]3([H])[C@@]1(C)C=CC(=O)N2
InChI
1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27)/t14-,15-,16-,17+,18+,22-,23+/m0/s1
InChI 密鑰
DBEPLOCGEIEOCV-WSBQPABSSA-N
基因資訊
human ... SRD5A2(6716)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Finasteride EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
选择性 5α-还原酶抑制剂;抗雄激素。
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
客戶也查看了
Manlio A Goetzl et al.
Nature clinical practice. Urology, 3(8), 422-429 (2006-08-12)
Prostate cancer chemoprevention involves the use of natural and/or synthetic agents that inhibit or reverse the development of precancerous lesions or delay progression of these lesions to invasive disease. The recent completion of the first Phase III trial for prostate
James Tacklind et al.
The Cochrane database of systematic reviews, (10)(10), CD006015-CD006015 (2010-10-12)
Benign prostatic hyperplasia (BPH), a non-malignant enlargement of the prostate in aging men, can cause bothersome urinary symptoms (intermittency, weak stream, straining, urgency, frequency, incomplete emptying). Finasteride, a five-alpha reductase inhibitor (5ARI), blocks the conversion of testosterone to dihydrotestosterone, reduces
Aditya K Gupta et al.
The Journal of dermatological treatment, 25(2), 156-161 (2013-06-19)
In the light of post-marketing reports of persistent sexual dysfunction with the use of finasteride, analysis of the extent of risk associated with 5α-reductase inhibitor treatment for androgenetic alopecia (AGA) is warranted. This study sought to evaluate the efficacy of
José Manuel Mella et al.
Archives of dermatology, 146(10), 1141-1150 (2010-10-20)
Androgenetic alopecia is the most common form of alopecia in men. To determine the efficacy and safety of finasteride therapy for patients with androgenetic alopecia. MEDLINE, EMBASE, CINAHL, Cochrane Registers, and LILACS were searched for randomized controlled trials reported in
Sergio Vañó-Galván et al.
Journal of the American Academy of Dermatology, 70(4), 670-678 (2014-02-11)
To our knowledge, there are no large multicenter studies concerning frontal fibrosing alopecia (FFA) that could give clues about its pathogenesis and best treatment. We sought to describe the epidemiology, comorbidities, clinical presentation, diagnostic findings, and therapeutic choices in a
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務