SMB00958
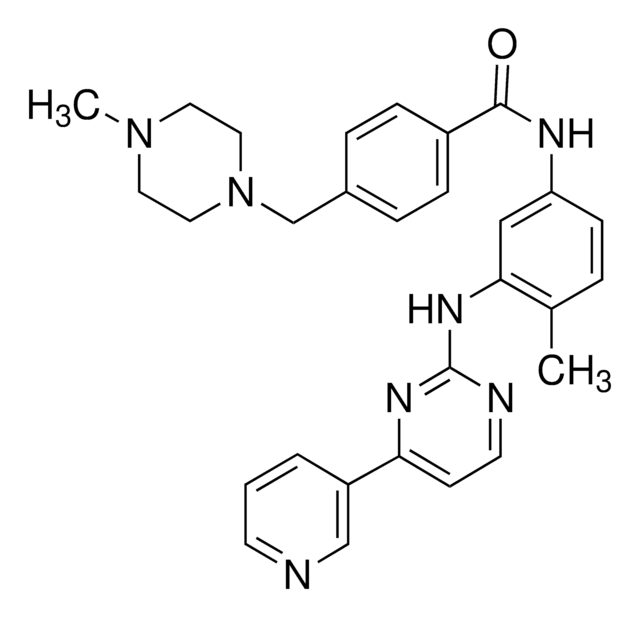
N-Desmethyl imatinib
≥95%
同義詞:
N-(4-Methyl-3-(4-(pyridin-3-yl)pyrimidin-2-ylamino)phenyl)-4-(piperazin-1-ylmethyl)benzamide, N-Desmethylimatinib, N-[4-Methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]-4-(1-piperazinylmethyl)benzamide, CGP74588, Desmethyl Gleevec, Norimatinib
About This Item
推薦產品
生物源
synthetic
品質等級
等級
research grade
化驗
≥95%
形狀
solid
分子量
479.58 g/mol
儲存條件
(Tightly closed. Dry. Keep in a well -ventilated place. Keep locked up or in an area accessible only to qualified or authorized persons.)
技術
HPLC: suitable
溶解度
ethanol: 0.20 mg/mL
DMF: 16 mg/mL
儲存溫度
2-8°C
InChI
1S/C28H29N7O/c1-20-4-9-24(17-26(20)34-28-31-12-10-25(33-28)23-3-2-11-30-18-23)32-27(36)22-7-5-21(6-8-22)19-35-15-13-29-14-16-35/h2-12,17-18,29H,13-16,19H2,1H3,(H,32,36)(H,31,33,34)
InChI 密鑰
BQQYXPHRXIZMDM-UHFFFAOYSA-N
一般說明
應用
其他說明
訊號詞
Danger
危險分類
Carc. 2 - Lact. - Muta. 2 - Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務