推薦產品
等級
pharmaceutical primary standard
API 家族
rifamycin, rifampicin
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
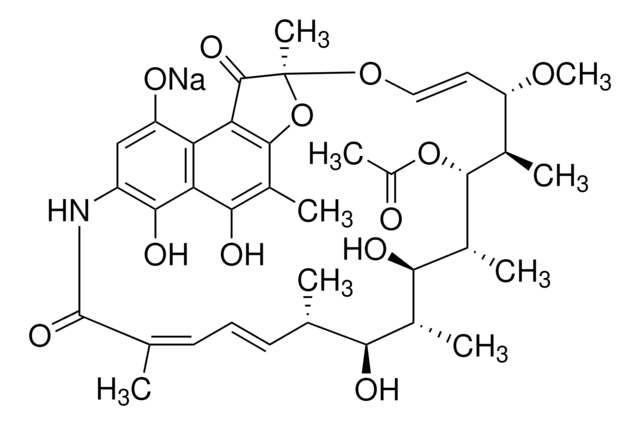
SMILES 字串
[Na+].CO[C@H]1\C=C\O[C@@]2(C)Oc3c(C)c(O)c4c(O)c(NC(=O)C(C)=C\C=C\[C@H](C)[C@H](O)[C@@H](C)[C@@H](O)[C@@H](C)[C@H](OC(C)=O)[C@@H]1C)cc([O-])c4c3C2=O
InChI
1S/C37H47NO12.Na/c1-16-11-10-12-17(2)36(46)38-23-15-24(40)26-27(32(23)44)31(43)21(6)34-28(26)35(45)37(8,50-34)48-14-13-25(47-9)18(3)33(49-22(7)39)20(5)30(42)19(4)29(16)41;/h10-16,18-20,25,29-30,33,40-44H,1-9H3,(H,38,46);/q;+1/p-1/b11-10+,14-13+,17-12-;/t16-,18+,19+,20+,25-,29-,30+,33+,37-;/m0./s1
InChI 密鑰
YVOFSHPIJOYKSH-NLYBMVFSSA-M
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Rifamycin sodium EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
A F D Di Stefano et al.
Antimicrobial agents and chemotherapy, 55(5), 2122-2128 (2011-03-16)
The new oral 200-mg rifamycin SV MMX modified-release tablets, designed to deliver rifamycin SV directly into the colonic lumen, offer considerable advantages over the existing immediate-release antidiarrheic formulations. In two pharmacokinetics studies of healthy volunteers, the absorption, urinary excretion, and
Zhihui Shao et al.
Archives of microbiology, 193(7), 463-477 (2011-03-23)
Nitrate assimilation has been well studied for Gram-negative bacteria but not so much in the Gram-positive actinomycetes up to date. In a rifamycin SV-producing actinomycete, Amycolatopsis mediterranei strain U32, nitrate not only can be used as a sole nitrogen source
Fatimata Seydou Sarr et al.
Journal of pharmaceutical and biomedical analysis, 52(1), 93-98 (2010-01-05)
In a previous paper, using a biophysical model system to study the passive diffusion of the statin molecules through the cell membrane, our group demonstrated that statins could cross biological membrane by passive diffusion (Sarr et al. [40]). However, in
Wei Zhao et al.
Cell research, 20(10), 1096-1108 (2010-06-23)
Amycolatopsis mediterranei is used for industry-scale production of rifamycin, which plays a vital role in antimycobacterial therapy. As the first sequenced genome of the genus Amycolatopsis, the chromosome of strain U32 comprising 10,236,715 base pairs, is one of the largest
Jan Magdalan et al.
Archives of toxicology, 83(12), 1091-1096 (2009-09-05)
The most often used antidote to treat poisoning caused by amanitin-containing mushrooms is benzylpenicillin (BPCN). However, a very few reports suggest that other antibiotics such as ceftazidime (CEFT) and rifamycin SV (RIFSV) show better antidote activity against amanitins than BPCN.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務