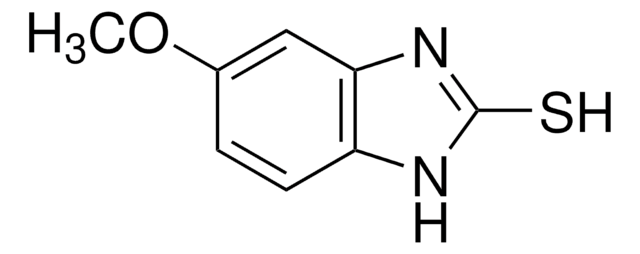
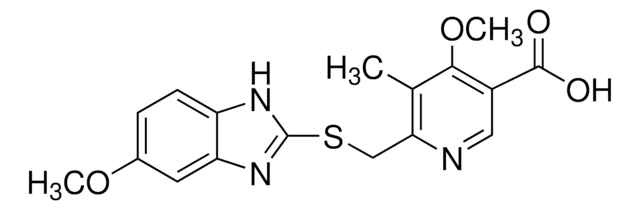
PHR1988
奥美拉唑杂质C
Pharmaceutical Secondary Standard; Certified Reference Material
同義詞:
Omeprazole Sulfide, 5-METHOXY-2-[[(4-METHOXY-3,5-DIMETHYLPYRIDIN-2-YL)METHYL]SULPHANYL]-1H-BENZIMIDAZOLE, 5-Methoxy-2-{[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]thio}-1H-benzimidazole, 6-Methoxy-2-{[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]thio}-1H-benzimidazole, Pyrmetazol, Ufiprazole
About This Item
推薦產品
等級
certified reference material
pharmaceutical secondary standard
品質等級
API 家族
omeprazole
CofA
current certificate can be downloaded
包裝
pkg of 30 mg
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
CC1=C(CSC2=NC3=CC(OC)=CC=C3N2)N=CC(C)=C1OC
InChI
1S/C17H19N3O2S/c1-10-8-18-15(11(2)16(10)22-4)9-23-17-19-13-6-5-12(21-3)7-14(13)20-17/h5-8H,9H2,1-4H3,(H,19,20)
InChI 密鑰
XURCIPRUUASYLR-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
應用
分析報告
其他說明
腳註
相關產品
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 2 - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務