O0151000
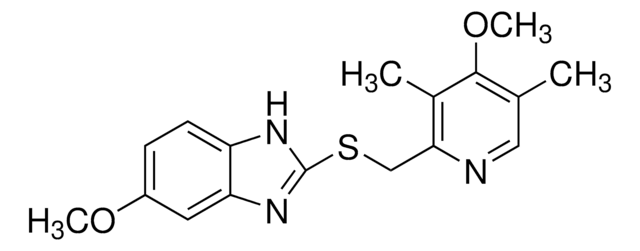
奥美拉唑杂质D
European Pharmacopoeia (EP) Reference Standard
同義詞:
Omeprazole sulfone, 5-Methoxy-2-{[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfonyl}-1H-benzimidazole, Omeprazole sulphone
登入查看組織和合約定價
全部照片(1)
About This Item
經驗公式(希爾表示法):
C17H19N3O4S
CAS號碼:
分子量::
361.42
Beilstein:
8347309
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推薦產品
等級
pharmaceutical primary standard
API 家族
omeprazole
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
O=S(C1=NC2=CC(OC)=CC=C2N1)(CC3=NC=C(C)C(OC)=C3C)=O
InChI
1S/C17H19N3O4S/c1-10-8-18-15(11(2)16(10)24-4)9-25(21,22)17-19-13-6-5-12(23-3)7-14(13)20-17/h5-8H,9H2,1-4H3,(H,19,20)
InChI 密鑰
IXEQEYRTSRFZEO-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Omeprazole impurity D EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
G García-Encina et al.
Journal of pharmaceutical and biomedical analysis, 21(2), 371-382 (2000-03-07)
An automated system using on-line solid-phase extraction and HPLC with UV detection has been validated in order to determine omeprazole in human plasma. The extraction was carried out using C18 cartridges. After washing, omeprazole was eluted from the cartridge with
Naser L Rezk et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 844(2), 314-321 (2006-08-22)
A simple, sensitive and specific reverse-phase high-performance liquid chromatography (HPLC) assay for the simultaneous quantitative determination of omeprazole and its three metabolites in human plasma was developed and validated. This method provides excellent chromatographic resolution and peak shape for the
Ia Hultman et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 848(2), 317-322 (2006-12-05)
A LC-MS/MS method was developed for quantitative determination of esomeprazole, and its two main metabolites 5-hydroxyesomeprazole and omeprazole sulphone in 25 microL human, rat or dog plasma. The analytes and their internal standards were extracted from plasma into methyl tert-butyl
Ylva Böttiger
European journal of clinical pharmacology, 62(8), 621-625 (2006-06-23)
The hydroxylation of omeprazole, measured as the ratio of omeprazole/5-hydroxyomeprazole in a plasma sample taken 3 h after an oral dose, is an established method to determine CYP2C19 activity, and the ratio of omeprazole AUC/omeprazole sulfone AUC has been used
A Abelö et al.
Drug metabolism and disposition: the biological fate of chemicals, 28(8), 966-972 (2000-07-20)
This study demonstrates the stereoselective metabolism of the optical isomers of omeprazole in human liver microsomes. The intrinsic clearance (CL(int)) of the formation of the hydroxy metabolite from S-omeprazole was 10-fold lower than that from R-omeprazole. However, the CL(int) value
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務