推薦產品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to Ph. Eur. F0285200
traceable to USP 1285750
API 家族
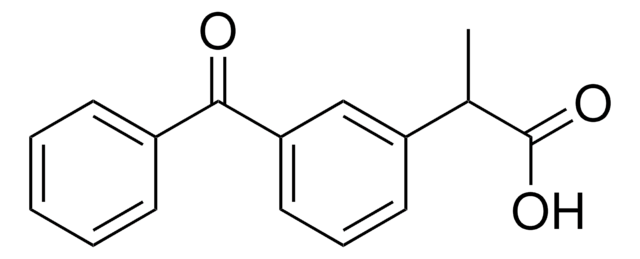
flurbiprofen
CofA
current certificate can be downloaded
包裝
pkg of 500 mg
技術
HPLC: suitable
gas chromatography (GC): suitable
mp
110-112 °C (lit.)
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-30°C
SMILES 字串
CC(C(O)=O)c1ccc(c(F)c1)-c2ccccc2
InChI
1S/C15H13FO2/c1-10(15(17)18)12-7-8-13(14(16)9-12)11-5-3-2-4-6-11/h2-10H,1H3,(H,17,18)
InChI 密鑰
SYTBZMRGLBWNTM-UHFFFAOYSA-N
基因資訊
human ... PTGS1(5742) , PTGS2(5743)
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Flurbiprofen is a non-selective, non-steroidal anti-inflammatory drug (NSAID) that is widely used against rheumatoid arthritis. It is also effective against vernal keratoconjunctivitis, post-operative ocular inflammation, herpetic stromal keratitis, excimer laser photorefractive keratectomy and ocular gingivitis.
Flurbiprofen is a non-selective, non-steroidal anti-inflammatory drug (NSAID) that is widely used against rheumatoid arthritis. It is also effective against vernal keratoconjunctivitis, post-operative ocular inflammation, herpetic stromal keratitis, excimer laser photorefractive keratectomy and ocular gingivitis.
應用
Flurbiprofen may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations and biological samples by various analytical techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
生化/生理作用
环氧合酶 (COX) 抑制剂;具有抗真菌活性的非甾体抗炎剂。S 对映体可抑制前列腺素的合成,同时具有消炎和镇痛活性,而 R 对映体不会抑制前列腺素的合成,只表现出镇痛活性。
Fluibiprofen is a cyclooxygenase (COX) inhibitor, which is an enzyme responsible for the conversion of arachidonic acid to prostaglandin G2 (PGG2) and PGG2 to prostaglandin H2 (PGH2) in the prostaglandin synthesis pathway. This decreases the prostaglandins which cause inflammation, pain, swelling and fever. Flurbiprofen inhibits the activity of both COX-1 and -2. The S enantiomer inhibits prostaglandin synthesis and has both anti-inflammatory and analgesic activity, while the R enantiomer does not inhibit prostaglandin synthesis and displays only analgesic activity.
分析報告
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAA3047 in the slot below. This is an example certificate only and may not be the lot that you receive.
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險聲明
防範說明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Determination of flurbiprofen in pharmaceutical formulations by UV spectrophotometry and liquid chromatography.
Sajeev C, et al.
Analytica Chimica Acta, 463(2), 207-217 (2002)
Sample pretreatment and determination of non steroidal anti-inflammatory drugs (NSAIDs) in pharmaceutical formulations and biological samples (blood, plasma, erythrocytes) by HPLC-UV-MS and μ-HPLC.
Mohamed S, et al.
Current Medicinal Chemistry, 12(5), 573-588 (2005)
The determination of non-steroidal antiinflammatory drugs in pharmaceuticals by capillary zone electrophoresis and micellar electrokinetic capillary chromatography.
Donato MG, et al.
Journal of Pharmaceutical and Biomedical Analysis, 12(1), 21-26 (1994)
Simultaneous Determination of Ceftriaxone Sodium and Non Steroidal Anti?Inflammatory Drugs in Pharmaceutical Formulations and Human Serum by RP?HPLC.
Sultana N, et al.
J. Chin. Chem. Soc., 57(6), 1278-1285 (2010)
Philippe Denis et al.
Investigative ophthalmology & visual science, 56(2), 1089-1096 (2015-01-22)
To evaluate the safety and efficacy of high-intensity focused ultrasound (HIFU) cyclocoagulation in reducing intraocular pressure (IOP) in patients with refractory glaucoma by using a novel miniaturized delivery device (EyeOP1). We conducted a 12-month open-label multicenter prospective study (EyeMUST1 Study).
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務