推薦產品
等級
pharmaceutical primary standard
API 家族
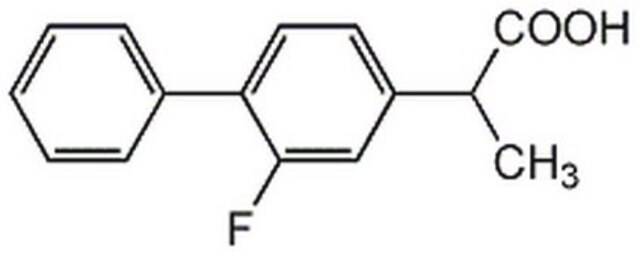
flurbiprofen
製造商/商標名
EDQM
mp
110-112 °C (lit.)
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
CC(C(O)=O)c1ccc(c(F)c1)-c2ccccc2
InChI
1S/C15H13FO2/c1-10(15(17)18)12-7-8-13(14(16)9-12)11-5-3-2-4-6-11/h2-10H,1H3,(H,17,18)
InChI 密鑰
SYTBZMRGLBWNTM-UHFFFAOYSA-N
基因資訊
human ... PTGS1(5742) , PTGS2(5743)
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Flurbiprofen EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
环氧合酶 (COX) 抑制剂;具有抗真菌活性的非甾体抗炎剂。S 对映体可抑制前列腺素的合成,同时具有消炎和镇痛活性,而 R 对映体不会抑制前列腺素的合成,只表现出镇痛活性。
Fluibiprofen is a cyclooxygenase (COX) inhibitor, which is an enzyme responsible for the conversion of arachidonic acid to prostaglandin G2 (PGG2) and PGG2 to prostaglandin H2 (PGH2) in the prostaglandin synthesis pathway. This decreases the prostaglandins which cause inflammation, pain, swelling and fever. Flurbiprofen inhibits the activity of both COX-1 and -2. The S enantiomer inhibits prostaglandin synthesis and has both anti-inflammatory and analgesic activity, while the R enantiomer does not inhibit prostaglandin synthesis and displays only analgesic activity.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Hugo Geerts
IDrugs : the investigational drugs journal, 10(2), 121-133 (2007-02-08)
(R)-flurbiprofen, the R-enantiomer of racemic flurbiprofen, is undergoing development by Myriad Genetics Inc, under license from Encore Pharmaceuticals Inc, for the potential treatment of Alzheimer's disease (AD). Devoid of any direct cyclooxygenase inhibition, which is associated with the more toxic
R N Brogden et al.
Drugs, 18(6), 417-438 (1979-12-01)
Flurbiprofen, a phenylalkanoic acid derivative, is a non-steroidal anti-inflammatory, antipyretic, analgesic agent advocated for use in rheumatoid arthritis, degenerative joint disease, ankylosing spondylitis and allied conditions. Published data suggest that flurbiprofen 120 to 150 mg daily is comparable in effectiveness
Laura Gasparini et al.
Brain research. Brain research reviews, 48(2), 400-408 (2005-04-27)
Currently, there is an intense debate on the potential use of nonsteroidal anti-inflammatory drugs (NSAIDs) in Alzheimer's disease (AD). NSAIDs are among the most widely prescribed drugs for the treatment of pain, fever, and inflammation. Their effects are largely attributed
N M Davies
Clinical pharmacokinetics, 28(2), 100-114 (1995-02-01)
Flurbiprofen is a chiral nonsteroidal anti-inflammatory drug (NSAID) of the 2-arylpropionic acid class. Although it possesses a chiral centre, with the S-(+)-enantiomer possessing most of the beneficial anti-inflammatory activity, both enantiomers may possess analgesic activity and all flurbiprofen preparations to
F Richy et al.
International journal of clinical practice, 61(8), 1396-1406 (2007-06-29)
The withdrawal of certain cyclooxygenase-2 selective drugs and the availability of over-the-counter non-steroidal anti-inflammatory drugs (NSAIDs) have increased the pressure for researching and prescribing conventional NSAIDs with a favourable efficacy/tolerance ratio in inflammatory diseases, particularly rheumatoid arthritis. The aim of
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務