PHR1389
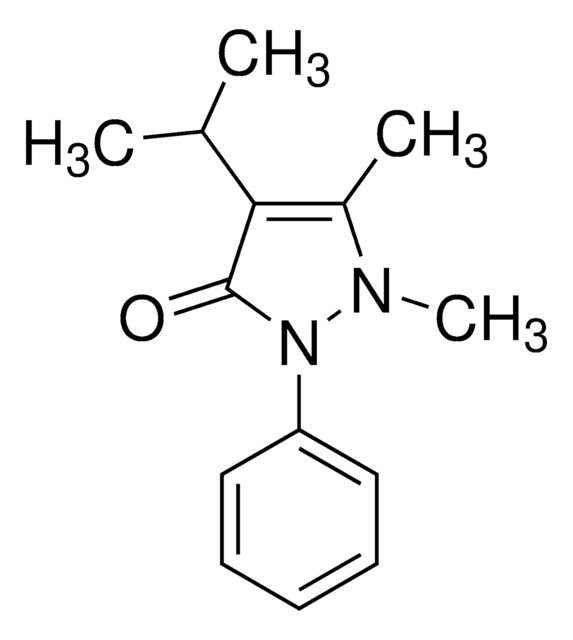
异丙安替比林
Pharmaceutical Secondary Standard; Certified Reference Material
同義詞:
4-Isopropylantipyrine, 1,2-Dihydro-1,5-dimethyl-4-(1-methylethyl)-2-phenyl-3H-pyrazol-3-one, 1,2-Dihydro-4-isopropyl-1,5-dimethyl-2-phenyl-3H-pyrazol-3-one, Isopropylphenazone, Propyphenazone
登入查看組織和合約定價
全部照片(1)
About This Item
經驗公式(希爾表示法):
C14H18N2O
CAS號碼:
分子量::
230.31
Beilstein:
204533
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推薦產品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to Ph. Eur. P3750000
API 家族
propyphenazone
CofA
current certificate can be downloaded
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
O=C1C(C(C)C)=C(C)N(C)N1C2=CC=CC=C2
InChI
1S/C14H18N2O/c1-10(2)13-11(3)15(4)16(14(13)17)12-8-6-5-7-9-12/h5-10H,1-4H3
InChI 密鑰
PXWLVJLKJGVOKE-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
Propyphenazone is a nonsteroidal drug exhibiting anti-inflammatory properties. It is widely used as a mild anesthetic medicine. It belongs to the pyrazolone class of compounds and has been posited to elicit an allergic or pseudo allergic reaction.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
應用
Propyphenazone can be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by chromatographic and spectrophotometric techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
分析報告
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAA1896 in the slot below. This is an example certificate only and may not be the lot that you receive.
A rapid spectrophotometric method to resolve ternary mixtures of propyphenazone, caffeine, and acetaminophen in tablets
Ozgur MU, et al.
Monatshefte fur Chemie / Chemical Monthly, 133(2), 219-223 (2002)
High-performance Liquid Chromatographic Determination of Paracetamol, Propyphenazone, and Caffeine in Pharmaceutical Formulations
Delvadiya K, et al.
Indian Journal of Pharmaceutical Education and Research, 47(4), 65-72 (2014)
Derivative ratio spectra-zero crossing spectrophotometry and LC method applied to the quantitative determination of paracetamol, propyphenazone and caffeine in ternary mixtures
Dinc E, et al.
Journal of Pharmaceutical and Biomedical Analysis, 26(5-6), 769-778 (2001)
Simultaneous determination of paracetamol, caffeine and propyphenazone in ternary mixtures by micellar electrokinetic capillary chromatography
Emre D and Ozalt?n, Nuran
Journal of Chromatography. B, Biomedical Sciences and Applications, 847(2), 126-132 (2007)
Simultaneous quantitation of paracetamol, caffeine and propyphenazone by high-pressure liquid chromatography
Mamolo MG, et al.
Journal of Pharmaceutical and Biomedical Analysis, 3(2), 157-164 (1985)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務