推薦產品
等級
pharmaceutical primary standard
API 家族
piperacillin
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
−20°C
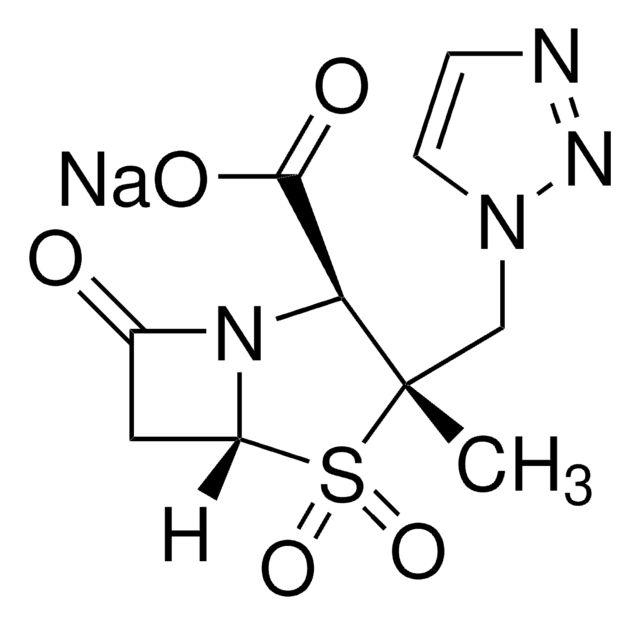
SMILES 字串
S1[C@H]2N([C@H](C1(C)C)C(=O)O)C(=O)[C@H]2NC(=O)[C@H](NC(=O)N4CCN(C(=O)C4=O)CC)c3ccccc3.O
InChI
1S/C23H27N5O7S.H2O/c1-4-26-10-11-27(19(32)18(26)31)22(35)25-13(12-8-6-5-7-9-12)16(29)24-14-17(30)28-15(21(33)34)23(2,3)36-20(14)28;/h5-9,13-15,20H,4,10-11H2,1-3H3,(H,24,29)(H,25,35)(H,33,34);1H2/t13-,14-,15+,20-;/m1./s1
InChI 密鑰
NBXPLBPWMYNZTC-IDYPWDAWSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Piperacillin EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Warning
危險聲明
危險分類
Aquatic Chronic 3 - Skin Sens. 1B
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Sangil Jeon et al.
Antimicrobial agents and chemotherapy, 58(7), 3744-3751 (2014-04-23)
Piperacillin in combination with tazobactam, a β-lactamase inhibitor, is a commonly used intravenous antibiotic for the empirical treatment of infection in intensive care patients, including burn patients. The purpose of this study was to develop a population pharmacokinetic (PK) model
Ashley W Sturm et al.
Pharmacotherapy, 34(1), 28-35 (2013-07-19)
To evaluate the steady-state pharmacokinetic and pharmacodynamic parameters of piperacillin in morbidly obese, surgical intensive care patients. Open-label single-center prospective study. Level I trauma center and university-affiliated teaching institution. Nine morbidly obese (body mass index [BMI] 40.0 kg/m² or higher)
Brian D VanScoy et al.
Antimicrobial agents and chemotherapy, 58(10), 6024-6031 (2014-07-30)
It is important to understand the relationship between antibiotic exposure and the selection of drug resistance in the context of therapy exposure. We sought to identify the ceftolozane-tazobactam exposure necessary to prevent the amplification of drug-resistant bacterial subpopulations in a
Raquel Cavalcanti Dantas et al.
Journal of medical microbiology, 63(Pt 12), 1679-1687 (2014-09-28)
The rates of multidrug-resistant, extensively drug-resistant and pandrug-resistant isolates amongst non-fermenting Gram-negative bacilli, particularly Pseudomonas aeruginosa, have risen worldwide. The clinical consequence of resistance and the impact of adverse treatment on the outcome of patients with P. aeruginosa bacteraemia remain
Jessica Krezdorn et al.
Journal of medical microbiology, 63(Pt 7), 945-955 (2014-06-15)
The aim of this study was to compare the inhibitory effect of antibiotic combinations in vitro with efficacy in Galleria mellonella larvae in vivo to identify efficacious combinations that target Pseudomonas aeruginosa. P. aeruginosa NCTC 13437, a multidrug-resistant strain resistant
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務