推薦產品
等級
pharmaceutical primary standard
API 家族
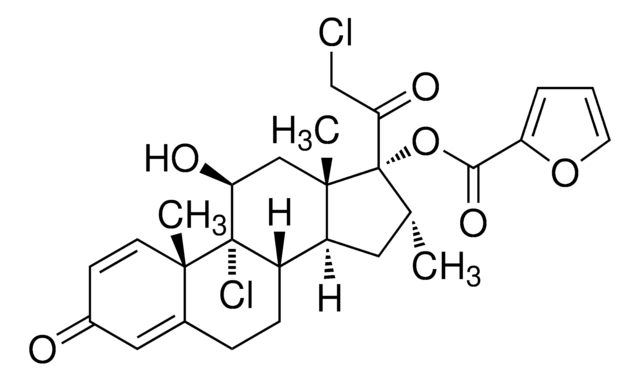
mometasone
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
C[C@@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@]4(C)[C@@]3(Cl)[C@@H](O)C[C@]2(C)[C@@]1(OC(=O)c5ccco5)C(=O)CCl
InChI
1S/C27H30Cl2O6/c1-15-11-19-18-7-6-16-12-17(30)8-9-24(16,2)26(18,29)21(31)13-25(19,3)27(15,22(32)14-28)35-23(33)20-5-4-10-34-20/h4-5,8-10,12,15,18-19,21,31H,6-7,11,13-14H2,1-3H3/t15-,18+,19+,21+,24+,25+,26+,27+/m1/s1
InChI 密鑰
WOFMFGQZHJDGCX-ZULDAHANSA-N
基因資訊
human ... NR3C1(2908)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Mometasone furoate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
Mometasone furoate is an anti-inflammatory glucocorticoid.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Mohamed A Bitar et al.
European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery, 270(3), 931-937 (2012-09-27)
This study aimed at observing the efficacy of mometasone fuorate monohydrate nasal spray on obstructive adenoids in children and identifying the characteristics of responders using a pilot study including children aged 2-11 years, with evidence of more than 50 %
Environmental exposure unit: a sensitive, specific, and reproducible methodology for allergen challenge.
Anne K Ellis et al.
Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology, 111(5), 323-328 (2013-10-16)
Johanna Svensson et al.
Primary care respiratory journal : journal of the General Practice Airways Group, 21(4), 412-418 (2012-09-25)
Acute rhinosinusitis is a common disease with an increasing incidence rate. It causes substantial costs to the individual and to society through healthcare consumption and absence from work. The use of antibiotics is widespread in the treatment of acute rhinosinusitis
Virat Kirtsreesakul et al.
American journal of rhinology & allergy, 26(6), 455-462 (2012-12-13)
Although combined oral and nasal steroid therapy is widely used in nasal polyposis, a subset of patients show an unfavorable therapeutic outcome. This study aimed to evaluate whether oral prednisolone produces any additive effects on subsequent nasal steroid therapy and
S-H Wu et al.
The British journal of dermatology, 168(1), 172-178 (2012-07-28)
Lipoxins are potential anti-inflammatory mediators and serve as an endogenous 'braking signal' in the inflammatory process. Accumulating evidence has indicated the efficacy of lipoxin A(4) (LXA(4) ) and its analogs in the treatment of many animal models of inflammatory diseases.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務