D1060000
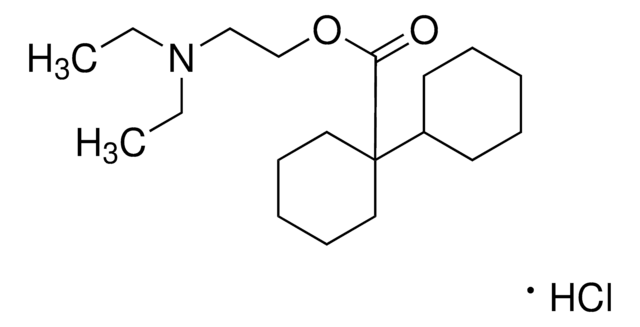
Dicycloverine hydrochloride
European Pharmacopoeia (EP) Reference Standard
同義詞:
Dicyclomine hydrochloride, 2-(Diethylamino)ethyl 1-cyclohexylcyclohexane-1-carboxylate hydrochloride, [1,1-Bicyclohexyl]-1-carboxylic acid 2-(diethylamino)ethyl ester hydrochloride
登入查看組織和合約定價
全部照片(1)
About This Item
經驗公式(希爾表示法):
C19H35NO2 · HCl
CAS號碼:
分子量::
345.95
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推薦產品
等級
pharmaceutical primary standard
API 家族
dicycloverine
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
CCN(CCOC(C1(C2CCCCC2)CCCCC1)=O)CC.[Cl]
InChI
1S/C19H35NO2.ClH/c1-3-20(4-2)15-16-22-18(21)19(13-9-6-10-14-19)17-11-7-5-8-12-17;/h17H,3-16H2,1-2H3;1H
InChI 密鑰
GUBNMFJOJGDCEL-UHFFFAOYSA-N
基因資訊
human ... CHRM1(1128) , CHRM3(1131)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Dicycloverine hydrochloride EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Recommendations for the management of Acanthamoeba keratitis.
Abdul Mannan Baig et al.
Journal of medical microbiology, 63(Pt 5), 770-771 (2014-02-11)
Brian L Le et al.
Research square (2021-04-07)
The novel SARS-CoV-2 virus emerged in December 2019 and has few effective treatments. We applied a computational drug repositioning pipeline to SARS-CoV-2 differential gene expression signatures derived from publicly available data. We utilized three independent published studies to acquire or
Gideon Koren
American journal of obstetrics and gynecology, 211(6), 602-606 (2014-08-26)
Presently, 97.7% of prescriptions for the treatment of nausea and vomiting in pregnancy in the United States are with medications not labeled for use in pregnancy, not indicated for nausea and vomiting in pregnancy, and not classified as safe in
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務