推薦產品
等級
pharmaceutical primary standard
agency
BP
API 家族
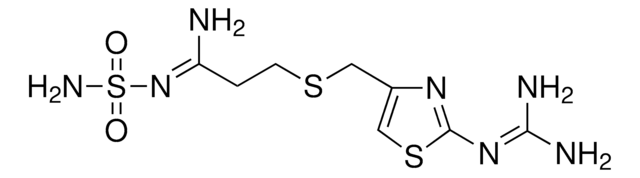
famotidine
形狀
solid
儲存期限
limited shelf life, expiry date on the label
包裝
pkg of 100 mg
製造商/商標名
BP
儲存條件
OK to freeze
顏色
white
mp
162-165 °C
溶解度
water: insoluble
應用
pharmaceutical
pharmaceutical small molecule
形式
neat
運輸包裝
ambient
儲存溫度
2-8°C
SMILES 字串
N\C(N)=N\c1nc(CSCCC(=N)NS(N)(=O)=O)cs1
InChI
1S/C8H15N7O2S3/c9-6(15-20(12,16)17)1-2-18-3-5-4-19-8(13-5)14-7(10)11/h4H,1-3H2,(H2,9,15)(H2,12,16,17)(H4,10,11,13,14)
InChI 密鑰
XUFQPHANEAPEMJ-UHFFFAOYSA-N
基因資訊
human ... HRH2(3274)
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
Famotidine is a hydrophilic, cationic, histamine H2 receptor antagonist drug that effectively inhibits gastric acid secretion in humans.
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Famotidine BP Reference standard, intended for use in laboratory tests only as specifically prescribed in the British Pharmacopoeia.
Also used in monographs such as:Famotidine Tablets
Also used in monographs such as:
生化/生理作用
H2组胺受体拮抗剂;抗溃疡剂。
包裝
Unit quantity: 100 mg. Subject to change. The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity please visit British Pharmacopoeia
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Famotidine
British Pharmacopeia
British Pharmacopoeia, 29(1-2), 247-254 (2020)
Stability indicating method for famotidine in pharmaceuticals using porous graphitic carbon column
Helali N and Monser L
Journal of Separation Science, 31(2) (2008)
Capillary zone electrophoresis method for the determination of famotidine and related impurities in pharmaceuticals
Helali N, et al.
Talanta, 74(4) (2008)
Potentiometric determination of famotidine in pharmaceutical formulations
Ayad MM, et al.
Journal of Pharmaceutical and Biomedical Analysis, 29(1-2) (2002)
Determination of cimetidine, famotidine, and ranitidine hydrochloride in the presence of their sulfoxide derivatives in pure and dosage forms by high-performance thin-layer chromatography and scanning densitometry
Kelani KM, et al.
Journal of AOAC (Association of Official Analytical Chemists) International, 85(5), 1015-1020 (2002)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務