推薦產品
等級
analytical standard
品質等級
產品線
PESTANAL®
儲存期限
limited shelf life, expiry date on the label
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
agriculture
environmental
格式
neat
儲存溫度
2-8°C
SMILES 字串
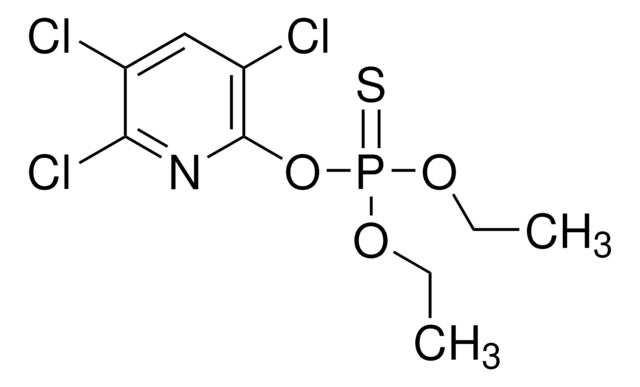
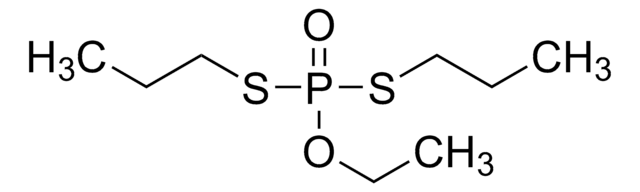
CCOP(=S)(OCC)SCCSCC
InChI
1S/C8H19O2PS3/c1-4-9-11(12,10-5-2)14-8-7-13-6-3/h4-8H2,1-3H3
InChI 密鑰
DOFZAZXDOSGAJZ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
法律資訊
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 1 Dermal - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
271.4 °F
閃點(°C)
133 °C
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
條款
analytical standard; Tokuthion, technical grade, pkg of 50 mg; Crotoxyphos; Phorate; Azinphos-ethyl; Diazinon; Azinphos-methyl; Demeton-O; Dimethoate; Chlorpyrifos-methyl
US EPA Method 8270 (Appendix IX): GC Analysis of Semivolatiles on Equity®-5 (30 m x 0.25 mm I.D., 0.50 μm)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務