推薦產品
品質等級
化驗
≥98.5% (sum of enantiomers, GC)
形狀
liquid
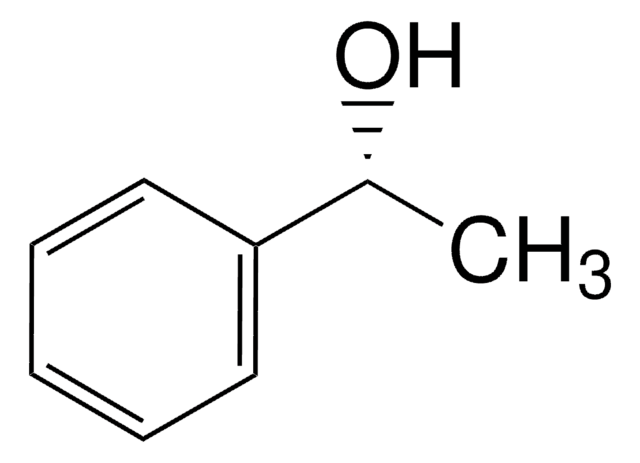
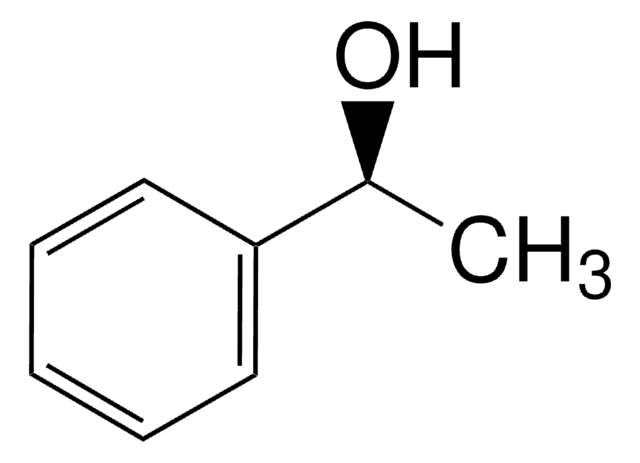
光學活性
[α]/D −45±2°, c = 5% in methanol
光學純度
enantiomeric ratio: ≥97:3 (GC)
折射率
n20/D 1.527
bp
88-89 °C/10 mmHg (lit.)
mp
9-11 °C (lit.)
密度
1.012 g/mL at 20 °C (lit.)
官能基
hydroxyl
phenyl
SMILES 字串
C[C@H](O)c1ccccc1
InChI
1S/C8H10O/c1-7(9)8-5-3-2-4-6-8/h2-7,9H,1H3/t7-/m0/s1
InChI 密鑰
WAPNOHKVXSQRPX-ZETCQYMHSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
(S)-(-)-1-Phenylethanol can be prepared from acetophenone via enantioselective bioreduction in the presence of Rhizopus arrhizus as a biocatalyst.
應用
(S)-(-)-1-Phenylethanol can be used as:
- A starting material to prepare (1S,3R,4S)-1-methyl-3,4-diphenyl-3,4-dihydro-1H-isochromene-3,4-diol (a cyclic hemiacetal) by reacting with benzil via dilithiation reaction.
- A chiral solvent in the symmetric synthesis of substituted spiroundecenetriones via amino acid-catalyzed domino Knoevenagel/Diels-Alder reactions.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
水污染物質分類(WGK)
WGK 3
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Laboratory scale-up synthesis of chiral carbinols using Rhizopus arrhizus
Salvi, NA and Chattopadhyay S
Tetrahedron Asymmetry, 27(4-5), 188-192 (2016)
Organocatalytic Asymmetric Domino Knoevenagel/Diels-Alder Reactions: A Bioorganic Approach to the Diastereospecific and Enantioselective Construction of Highly Substituted Spiro [5, 5] undecane-1, 5, 9-triones
Ramachary DB, et al.
Angewandte Chemie (International Edition in English), 42(35), 4233-4237 (2003)
文章
Chiral Alcohols
Chiral Alcohols
Chromatograms
suitable for GC我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務