Chiral Alcohols
We offer a wide range of chiral alcohols to meet their ever-growing demand. These useful reagents may serve both as starting materials in the synthesis of single-stereoisomer drugs or intermediates, or as powerful resolving agents.
2-Hexanol
(R)-(–)-2-Hexanol and (S)-(+)-2-hexanol were used in the preparation of some key intermediates for model studies in the total synthesis of antivirally active glycolipid cycloviracin B1 (Scheme 1). By comparing the NMR chemical shifts of the synthesized model compounds with the ones of the isolated natural product, configurations of four previously unassigned stereocenters of cycloviracin B1 could be elucidated.
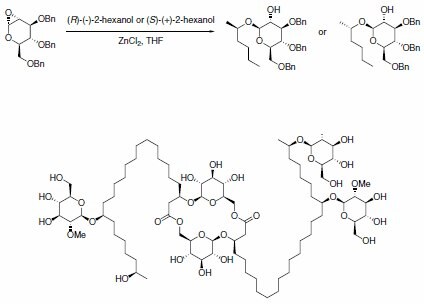
Scheme 1.Hexanol
2-Heptanol
The (R)-enantiomer of 2-heptanol was used by Kondo et al. in resolving the diastereoisomeric mixture of a key intermediate in the synthesis of 1-(2-chloro-4-pyrrolidin-1-ylbenzoyl)-2,3,4,5-tetrahydro-1H-1-benzdiazepine, known to be a strong vasopressin V2 receptor agonist, which helps to maintain normal plasma osmolality, blood volume, and blood pressure (Scheme 2). A nonpeptidic V2 agonist may find use in the treatment of diabetes insipidus and nocturnal enuresis.
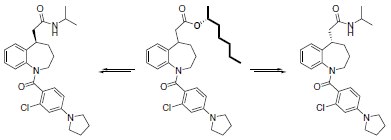
Scheme 2.Heptanol
References
To continue reading please sign in or create an account.
Don't Have An Account?