推薦產品
品質等級
化驗
≥98.0% (HPLC)
形狀
powder or crystals
應用
food and beverages
metabolomics
vitamins, nutraceuticals, and natural products
儲存溫度
2-8°C
SMILES 字串
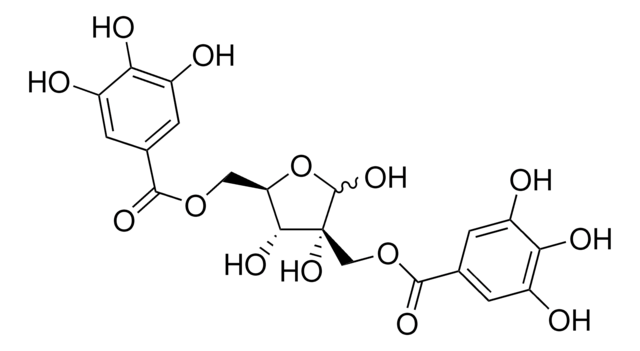
OC1O[C@H](COC(=O)c2cc(O)c(O)c(O)c2)[C@@H](O)[C@]1(O)COC(=O)c3cc(O)c(O)c(O)c3
InChI
1S/C20H20O14/c21-9-1-7(2-10(22)14(9)25)17(28)32-5-13-16(27)20(31,19(30)34-13)6-33-18(29)8-3-11(23)15(26)12(24)4-8/h1-4,13,16,19,21-27,30-31H,5-6H2/t13-,16-,19?,20-/m1/s1
InChI 密鑰
FEPAFOYQTIEEIS-IZUGRSKYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Hamamelitanninis a tannin derived from the bark and leaves of Hamamelis virginiana(witch hazel).
應用
Hamamelitannin can be used to study chromatography, aromatics, esters, hamamelis, heterocyclics, natural compounds, phenols, phytopharma standards, polyhydroxy compounds, and tannins. It also significantly reduces biofilm metabolic activity of the following bacteria: Staphylococcus epidermidis, Staphylococcus aureus, Acinetobacter baumannii, and Candida albicans strains. Hamamelitannin displays specific cytotoxic activity against colon cancer cells. It has been used in a study to determine that quorum-sensing inhibitors increase the success of antibiotic treatment by increasing the susceptibility of bacterial biofilms and/or by increasing host survival following infection.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
E Okochi et al.
Biological & pharmaceutical bulletin, 18(1), 49-52 (1995-01-01)
N-Nitrosodialkylamines are environmental alkylating carcinogens which are metabolically activated to alpha-hydroxy nitrosamines by cytochrome P450. The precise mechanism of their activation is not well understood, and a simplified chemical model for cytochrome P450 as a non-enzymatic system is useful for
Hye Rhi Choi et al.
Phytotherapy research : PTR, 16(4), 364-367 (2002-07-12)
Peroxynitrite (ONOO(-)) is a cytotoxicant with strong oxidizing properties toward various cellular constituents, including sulphydryls, lipids, amino acids and nucleotides and can cause cell death, lipid peroxidation, carcinogenesis and aging. The aim of this study was to characterize ONOO(-) scavenging
Andreas Dauer et al.
Phytochemistry, 63(2), 199-207 (2003-04-25)
The genotoxic and antigenotoxic activities of catechin, hamamelitannin and two proanthocyanidin fractions prepared from the bark of Hamamelis virginiana L. were investigated in a human derived, metabolically competent hepatoma cell line (Hep G2) using single cell gel electrophoresis (SCGE) for
L Cobrado et al.
The Journal of antimicrobial chemotherapy, 67(5), 1159-1162 (2012-02-10)
The colonization of indwelling medical devices and subsequent biofilm formation represents a global challenge since it promotes the persistence of infection and contributes to antimicrobial resistance. The aim of this study was to determine the antimicrobial activity of cerium, chitosan
K Saeki et al.
Planta medica, 65(3), 227-229 (1999-05-08)
Tetragalloylglucose (TgG) and digalloylhamamelose (DgH) were found to inhibit adhesion to and invasion through Matrigel of mouse Lewis lung carcinoma LL2-Lu3 cells, which are highly metastatic. TgG inhibited matrix metalloproteinases (MMPs) from the tumor cells like (-)-epigallocatechin gallate, whereas DgH
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務