推薦產品
生物源
rabbit
抗體表格
affinity isolated antibody
抗體產品種類
primary antibodies
無性繁殖
polyclonal
物種活性
human
技術
inhibition assay: suitable (peptide)
western blot: suitable
同型
IgG
NCBI登錄號
UniProt登錄號
目標翻譯後修改
phosphorylation (pSer390)
基因資訊
human ... LMNA(4000)
一般說明
Prelamin-A/C (UniProt: P02545) is encoded by the LMNA (also known as LMN1) gene (Gene ID: 4000) in human. Prelamin-A/C is subsequently cleaved into Lamin A/C. Lamins are components of the nuclear lamina that provides a framework for the nuclear envelope and interact with chromatin. Prelamin-A/C
Is cleaved to generate Lamin A/C. Farnesylation of prelamin-A/C facilitates nuclear envelope targeting and subsequent cleavage by ZMPSTE24/FACE1 to remove the farnesyl group produces mature Lamin-A/C that is inserted into the nuclear lamina. Lamin A and C are present in equal amounts in the lamina of mammals and they play an important role in nuclear assembly, chromatin organization, nuclear membrane and telomere dynamics. Lamins are shown to be essential for normal development of peripheral nervous system and skeletal muscle and for muscle satellite cell proliferation. Lamins also prevent fat infiltration of muscle and bone marrow, helping to maintain the volume and strength of skeletal muscle and bone. Phosphorylation of Lamins is reported to occur continuously throughout all interphase periods and takes place mainly on the assembled lamina. Phosphorylation of the major polypeptides of the lamina induces laminar disassembly during mitosis. Phosphorylated Lamin-A/C localizes to nucleoplasm. Lamin A/C undergoes phosphorylation at multiple sites and one of the best characterized phosphorylation sites is on Serine 22 and it is phosphorylated during interphase. Phosphorylation of Serine 22 stabilizes Lamin A/C. Overexpression of Lamin-A is shown to result in greater phosphorylation of Serine 22 and 390 and Lamin A/C knockdowns display reduced phosphorylation at both sites, which helps in maintaining the integrity of the diminished lamina. Mutations in LMNA gene can cause Emery-Dreifuss muscular dystrophy 2 and 3, which are characterized by weakness and atrophy of muscle without involvement of the nervous system and cardiac conduction defects. Some mutations have also been linked to familial Lipodystrophy that leads to the loss of subcutaneous adipose tissue in the lower parts of the body and accumulation of adipose tissue in the face and neck. (Ref.: Buxboim, A., et al. (2014). Curr. Biol. 24(16): 1909-1917).
Is cleaved to generate Lamin A/C. Farnesylation of prelamin-A/C facilitates nuclear envelope targeting and subsequent cleavage by ZMPSTE24/FACE1 to remove the farnesyl group produces mature Lamin-A/C that is inserted into the nuclear lamina. Lamin A and C are present in equal amounts in the lamina of mammals and they play an important role in nuclear assembly, chromatin organization, nuclear membrane and telomere dynamics. Lamins are shown to be essential for normal development of peripheral nervous system and skeletal muscle and for muscle satellite cell proliferation. Lamins also prevent fat infiltration of muscle and bone marrow, helping to maintain the volume and strength of skeletal muscle and bone. Phosphorylation of Lamins is reported to occur continuously throughout all interphase periods and takes place mainly on the assembled lamina. Phosphorylation of the major polypeptides of the lamina induces laminar disassembly during mitosis. Phosphorylated Lamin-A/C localizes to nucleoplasm. Lamin A/C undergoes phosphorylation at multiple sites and one of the best characterized phosphorylation sites is on Serine 22 and it is phosphorylated during interphase. Phosphorylation of Serine 22 stabilizes Lamin A/C. Overexpression of Lamin-A is shown to result in greater phosphorylation of Serine 22 and 390 and Lamin A/C knockdowns display reduced phosphorylation at both sites, which helps in maintaining the integrity of the diminished lamina. Mutations in LMNA gene can cause Emery-Dreifuss muscular dystrophy 2 and 3, which are characterized by weakness and atrophy of muscle without involvement of the nervous system and cardiac conduction defects. Some mutations have also been linked to familial Lipodystrophy that leads to the loss of subcutaneous adipose tissue in the lower parts of the body and accumulation of adipose tissue in the face and neck. (Ref.: Buxboim, A., et al. (2014). Curr. Biol. 24(16): 1909-1917).
特異性
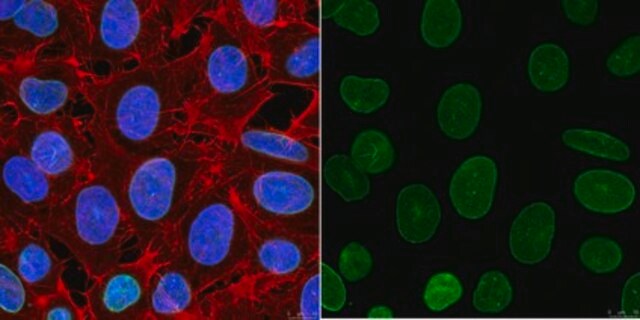
This rabbit polyclonal antibody detects human Lamin A/C phosphorylated on serine 390.
免疫原
KLH-conjugated linear peptide corresponding to 11 amino acids from human Lamin A/C surrounding phosphorylated Serine 390.
應用
Anti-Phospho-Lamin A/C (Ser390), Cat. No. ABT1388, is a rabbit polyclonal antibody that detects Lamin A/C phosphorylated on Serine 390 and has been tested for use in Western Blotting and Peptide Inhibition Assay.
Peptide Inhibition Analysis: A 1:500 dilution from a representative lot was used with A549 cells (specific for Lamin A _C phosphorylation) for peptide block analysis.
品質
Evaluated by Western Blotting in A549 cells.
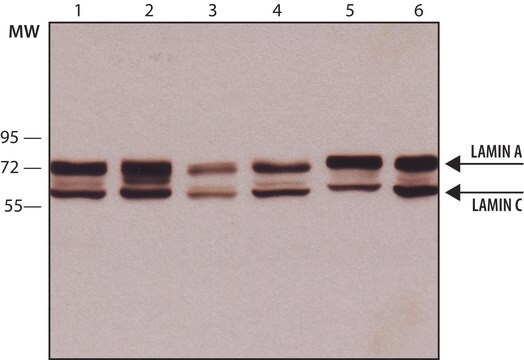
Western Blotting Analysis: A 1:500 dilution of this antibody detected Phospho-Lamin A/C (Ser390) in A549 cells (specific for Lamin A/C phosphorylation).
Western Blotting Analysis: A 1:500 dilution of this antibody detected Phospho-Lamin A/C (Ser390) in A549 cells (specific for Lamin A/C phosphorylation).
標靶描述
~75 kDa and 65 kDa observed; 74.14 and 65.14 kDa calculated for Lamin A and C, respectively. Uncharacterized bands may be observed in some lysate(s).
外觀
Format: Purified
其他說明
Concentration: Please refer to lot specific datasheet.
未找到適合的產品?
試用我們的產品選擇工具.
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務